Eye medicine for treating fungal keratitis
A technology for fungal keratitis and ophthalmic medicine, which is applied in the field of ophthalmic medicine for treating fungal keratitis, can solve the problems of lack of supply, poor antibacterial effect of ocular fungi, and difficulty in patients' promotion and use, and achieves simple preparation method and exact curative effect. , obvious curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation and detection of the eye medicine for the treatment of fungal keratitis
[0034] 1. Preparation of eye drops
[0035] Get terbinafine hydrochloride (by C 21 h 25 N) 2.5g, hydroxypropyl β-cyclodextrin 35g, dissolved in water for injection, then add 1.0mL of 5% benzalkonium bromide solution and 8g of NaCl, adjust the pH to 4.0-5.5 with 0.1M sodium hydroxide, Finally, add water for injection to make the volume to 1000mL, mix well, filter, and sterilize by flowing steam at 100°C for 30 minutes to obtain transparent eye drops for treating fungal keratitis, which are aseptically divided into 10mL bottles.
[0036] Terbinafine is insoluble in water and has certain irritation. Dissolution tests are carried out with various organic solvents such as acetone, ethanol, DMSO, methanol and chloroform. As a result, acetone, ethanol and DMSO dissolve the best, but they are not easy to use eyes. In the prescription research, different ratios of borax an...
Embodiment 2
[0058] Embodiment 2, the preparation of the eye medicine for the treatment of fungal keratitis
[0059] Get terbinafine hydrochloride (by C 21 h 25 N meter) 5g, β-cyclodextrin 25g, dissolved in water for injection, adjust pH to 4.0-5.5 with 0.1M sodium hydroxide, add water for injection to make up to 1000mL, mix well, filter, and circulate steam at 100°C Sterilize for 30 minutes to obtain transparent eye drops for treating fungal keratitis, which are aseptically divided into 10 mL bottles.
[0060] Detect with the method identical with embodiment 1, test result shows that every test result of ophthalmic medicine of the present invention all meets the regulations.
Embodiment 3
[0061] Embodiment 3, drug effect, pharmacology and toxicology analysis of eye drops of the present invention
[0062] 1. Efficacy test
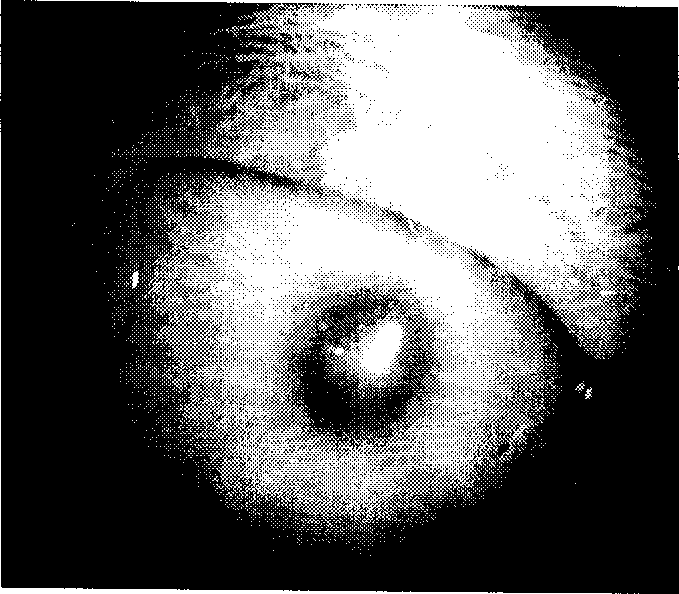
[0063] Isolate pathogenic fungus specimens from the eyes of patients with fungal keratitis, and dilute to 2-5×10 after passage identification 6 / mL concentration, inoculate the corneal stromal layer of rabbit eyes, the specific method is: select 30 rabbits of 1.5 kg, divide into 6 groups, 5 in every group (total 10 eyes), wherein three groups are treatment groups, use three groups respectively. Terbinafine eye drops of different concentrations were used for treatment; the other three were control groups, one of which was treated with 5% natezhen as a positive control, and the remaining two groups were solvent eye drops negative control group and model control without treatment. Group. The observation results after 2 days of treatment are as follows Figure 1A-1B As shown, the observation results after 12 days of treatment are as follows ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com