Voriconazole-coated carrageenan corneal contact lens and preparation method thereof
A technology of voriconazole and glue cornea, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of the preparation method of entrapped voriconazole that have not been reported, and improve treatment compliance Sex, reduce dosage, improve the effect of treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The components and contents of the voriconazole-embedded carrageenan contact lens involved in this embodiment are as follows: successively add silver salt solution and voriconazole solution to the carrageenan aqueous solution, and the mass volume ratio of the carrageenan aqueous solution to the silver salt solution and the voriconazole solution is: 10 grams: 5-15 microliters: 7-13 microliters, wherein the mass percentage concentration of the carrageenan aqueous solution is 0.99-10%, the voriconazole solution is the voriconazole DMSO solution with a mass concentration of 0.1-5 mg / microliter, silver salt solution Be the silver nitrate aqueous solution that molar concentration is 0.1 mol / liter; Concrete preparation process step comprises:
[0030] (1) Preparation of carrageenan solution: Weigh 0.099-1 gram of κ-carrageenan, add 9-9.901 grams of sterilized deionized water, heat in a water bath to 50-90°C, stir for 0.5-3 hours, and configure the mass percentage concentration ...
Embodiment 2
[0035] The components and contents of the voriconazole-embedded carrageenan contact lens involved in this embodiment are as follows: successively add silver salt solution and voriconazole solution to the carrageenan aqueous solution, and the mass volume ratio of the carrageenan aqueous solution to the silver salt solution and the voriconazole solution is: 10 grams: 5-15 microliters: 7-13 microliters, wherein the mass percentage concentration of the carrageenan aqueous solution is 0.99-10%, the voriconazole solution is the voriconazole DMSO solution with a mass concentration of 0.1-5 mg / microliter, silver salt solution Be the silver nitrate aqueous solution that molar concentration is 0.1 mol / liter; Concrete preparation process step comprises:
[0036] (1) Preparation of carrageenan solution: Weigh 0.3 g of κ-carrageenan, add 9.7 g of sterilized deionized water, heat in a water bath to 70° C., stir for 1 hour, and configure a carrageenan aqueous solution with a mass percentage c...
Embodiment 3
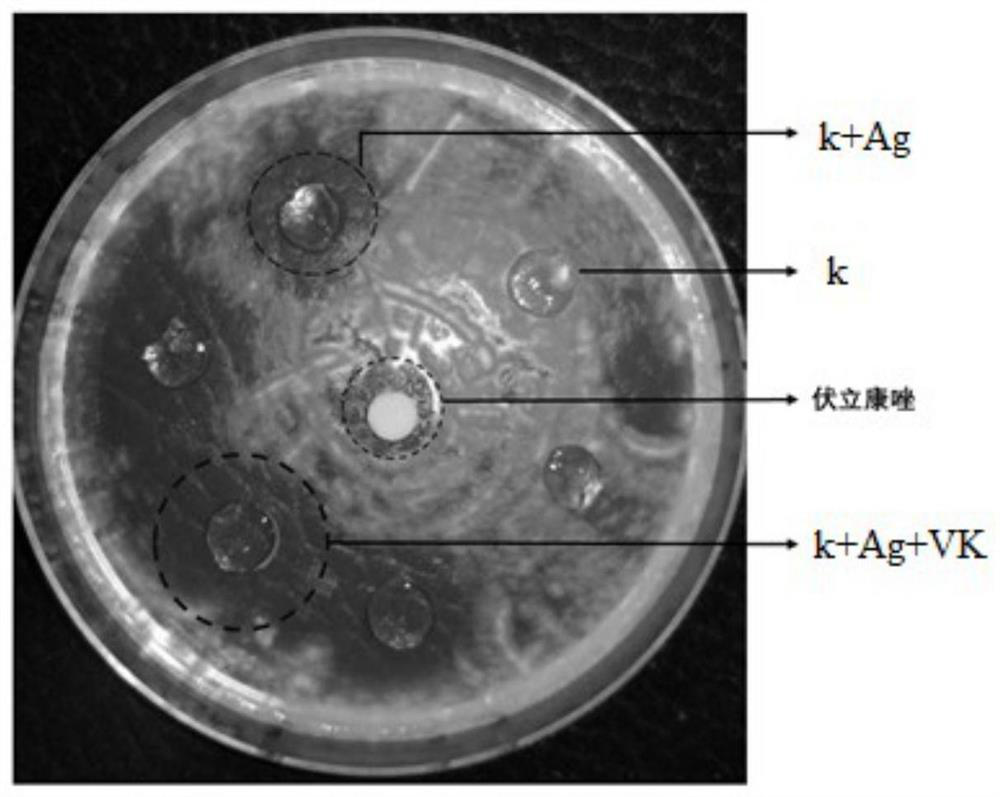
[0041] This example relates to an in vitro release test of a carrageenan contact lens embedded with voriconazole.
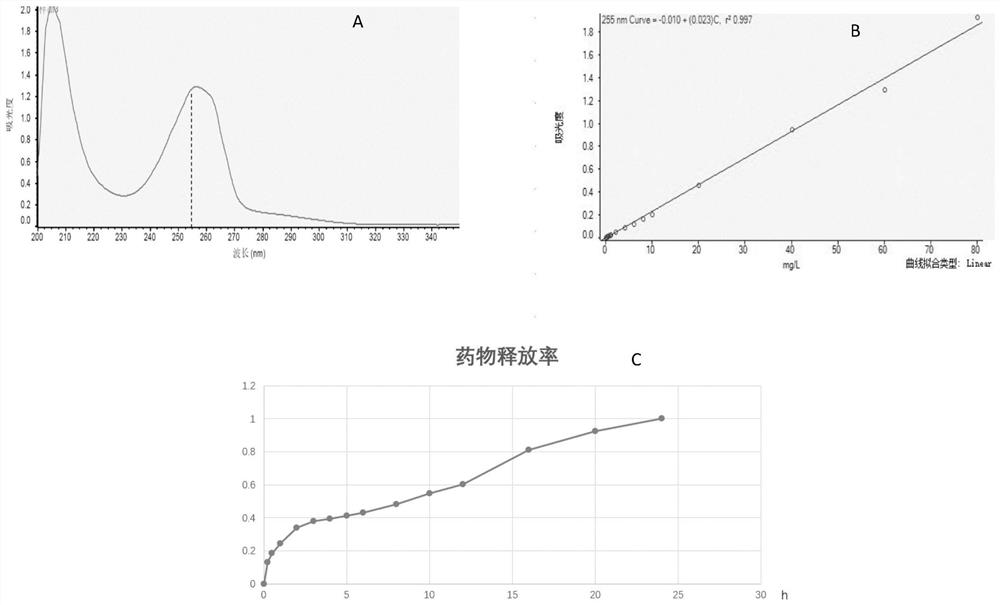
[0042] Step 1: draw the absorbance-concentration standard curve of voriconazole: first detect the ultraviolet absorption spectrum of voriconazole in the wavelength range of 200-350nm (such as figure 2 Shown in A), set 255nm as the voriconazole detection peak, then carry out the 255nm ultraviolet absorption peak measurement to the concentration gradient of 0 to 80 mg / liter voriconazole DMSO solution, draw the absorbance-concentration standard curve of voriconazole (such as figure 2 shown in B).
[0043] Step 2: Put 5 corneal contact membranes with a diameter of 14mm prepared in Example 2 into a dialysis bag (MWCO1000Da), completely immerse the dialysis bag in 100mL of PBS buffer solution (PH=7.4), and then rotate at a speed of 37 rpm , shake at a temperature of 37°C, take samples at the time points of shaking 0, 0.25h, 0.5h, 1h, 2h, 3h, 4h, 5h, 6h, 8h, 10h, 12h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com