Human keratinized cell growth factor-1 analogue preparation method and application thereof
A keratinocyte, growth factor technology, applied in the field of genetic engineering, can solve the problems of unsatisfactory KGF yield, high cost, time-consuming, etc., and achieves the effects of increasing activity, improving production efficiency, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
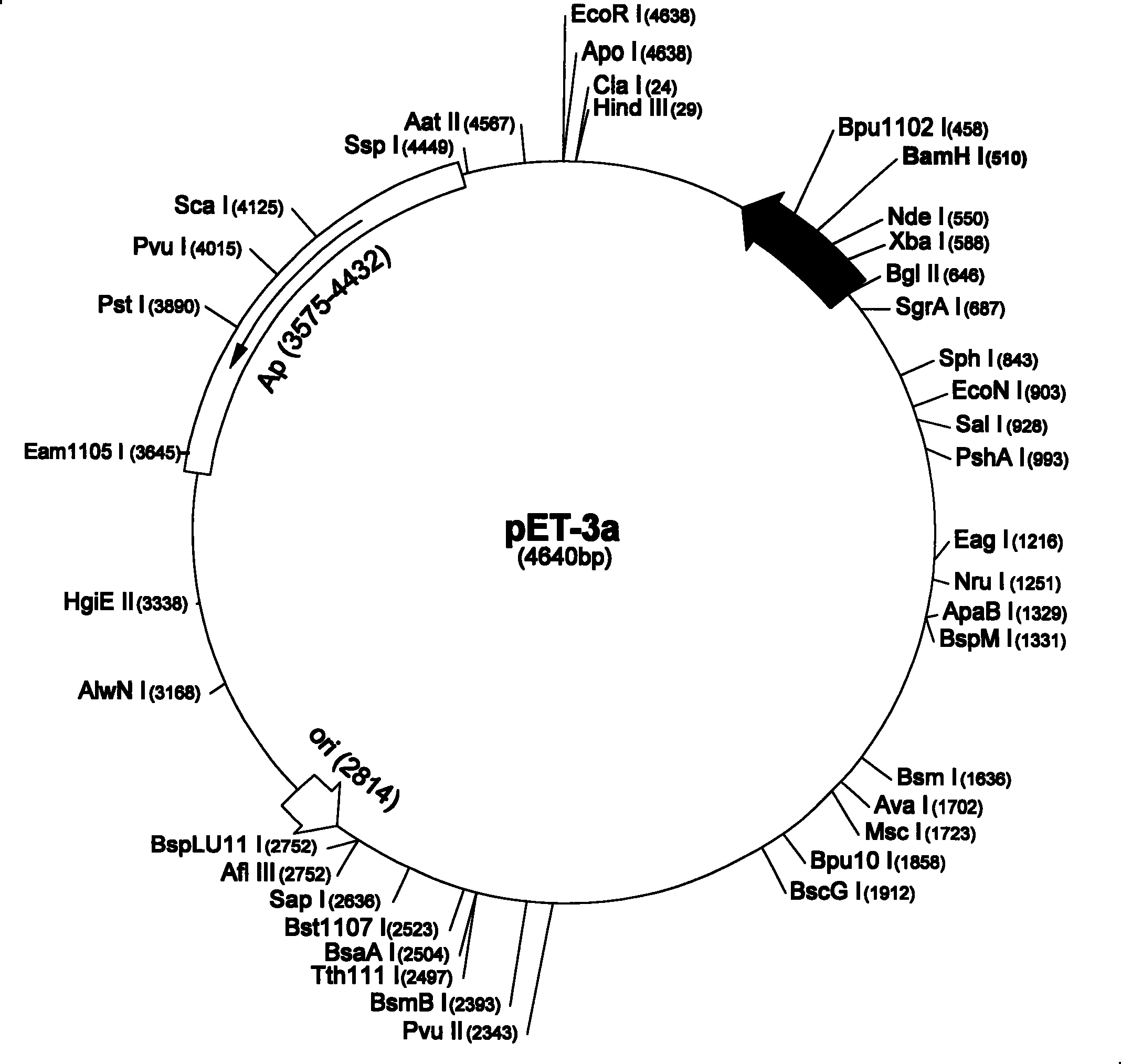
[0026] According to the nucleotide sequence (SEQ ID No.5) of human KGF1 on the NCBI network, its amino acid sequence is composed of 163 amino acids, as shown in (SEQ ID No.6), use DNAStar software to design and synthesize 13 primers, and apply the recursive PCR method Artificially synthesized the whole gene sequence of KGF, and designed a pair of primers at both ends of KGF to synthesize Δ23KGF, then designed a pair of primers at position 40, and mutated cysteine to serine to obtain Δ23KGF (40S). And remove the NdeI and BamH I sites contained inside it. Escherichia coli preferred codons were selected for artificial synthesis in order to increase the level of recombinant expression.
[0027] F1 (SEQ ID No. 9): GACATACCCGTAGCTATGATTATATGGAAGGTGGTGATATTCGTGTGCGTCGTCTGTTT (79-137)
F2 (SEQ ID No. 10): AACAGATGGCGACGAATGTGAATTGCAGCAGCCCAGAAAGACATACCCGTAGCTATGAT (40-98)
F3 (SEQ ID No. 11): ACAGAGAACAGATTGGTGGTTGTAATGATATGACCCCGGAACAGATGGCGACGAATGTG (1-59)
...
Embodiment 2
[0083] SEQ ID NO: 27: Ulp-F2 (5'-3', 59 bases in total)
[0084] SEQ ID NO: 28: Ulp-F3 (5'-3', 59 bases in total)
[0085] SEQ ID NO: 29: Ulp-F4 (5'-3', 59 bases in total)
[0086] SEQ ID NO: 30: Ulp-F5 (5'-3', 46 bases in total)
[0087] SEQ ID NO: 31: Ulp-F6 (5'-3', 33 bases in total)
[0088] SEQ ID NO: 32: Ulp-F7 (5'-3', 59 bases in total)
[0089] SEQ ID NO: 33: Ulp-F8 (5'-3', 59 bases in total)
[0090] SEQ ID NO: 34: Ulp-F9 (5'-3', 59 bases in total)
[0091] SEQ ID NO: 35: Ulp-R1 (5'-3', 59 bases in total)
[0092] SEQ ID NO: 36: Ulp-R2 (5'-3', 59 bases in total)
[0093] SEQ ID NO: 37: Ulp-R3 (5'-3', 59 bases in total)
[0094] SEQ ID NO: 38: Ulp-R4 (5'-3', 59 bases in total)
[0095] SEQ ID NO: 39: Ulp-R5 (5'-3', 59 bases in total)
[0096] SEQ ID NO: 40: Ulp-R6 (5'-3', 59 bases in total)
[0097] SEQ ID NO: 41: Ulp-R7 (5'-3', 59 bases in total)
[0098] SEQ ID NO: 42: Ulp-R8 (5'-3', 59 bases in total)
[0099] SEQ ID NO: 43: Ulp-R9 (5'-3', 59 bases in to...
Embodiment 3
[0108] As far as the tandem connection between the two transcription units is concerned, the recombinant expression vector of the present invention first includes a mature peptide encoding a small molecule ubiquitin-related modifier operatively connected to the first promoter, which is similar in structure to human KGF-1 The nucleotide sequence of the fusion protein composed of the substance, and an insertion site for an operable second transcription unit. With this insertion site, the second transcription unit consisting of the nucleotide sequence encoding the ubiquitin-associated modifier protease and the second promoter upstream thereof can be easily inserted into the expression vector.
[0109] In order to construct the cDNA sequence of the fusion protein containing the mature peptide encoding small molecule ubiquitin-related modification factors under the control of the T7 promoter and the human KGF-1 structural analogue connected in tandem under the control of the T7 prom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com