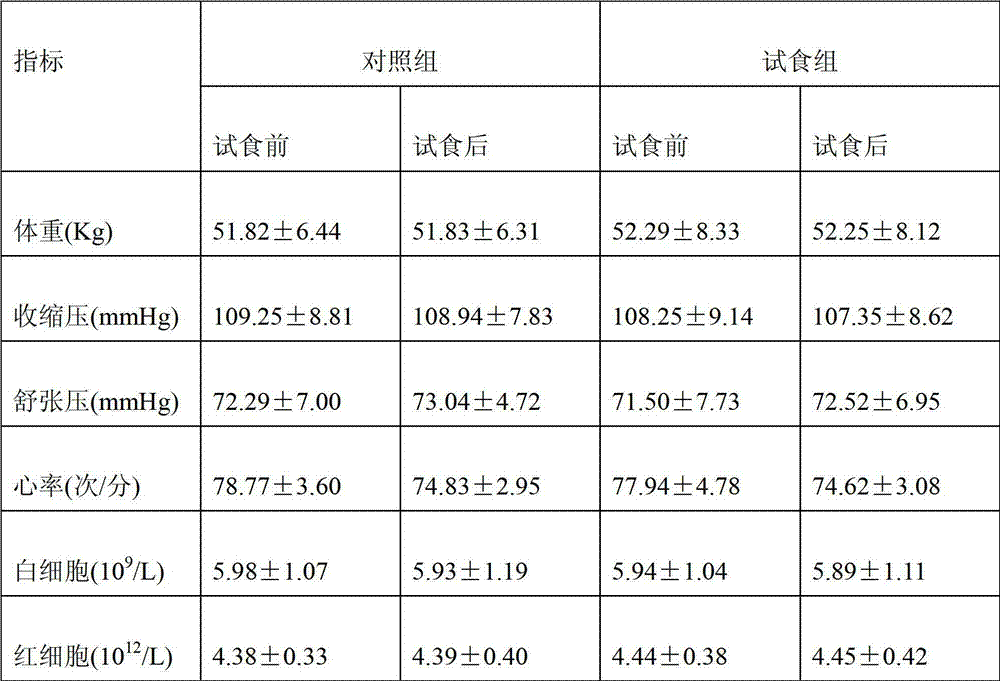
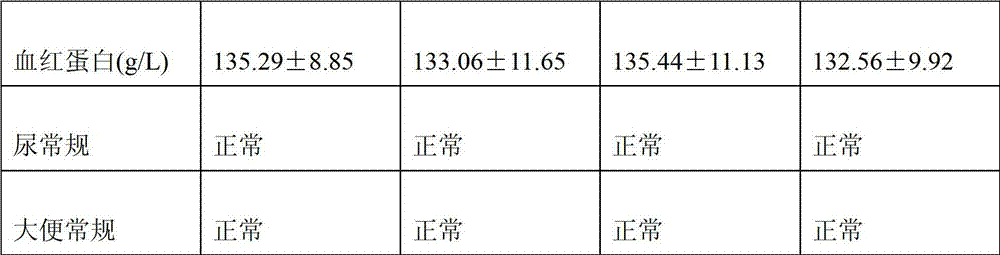
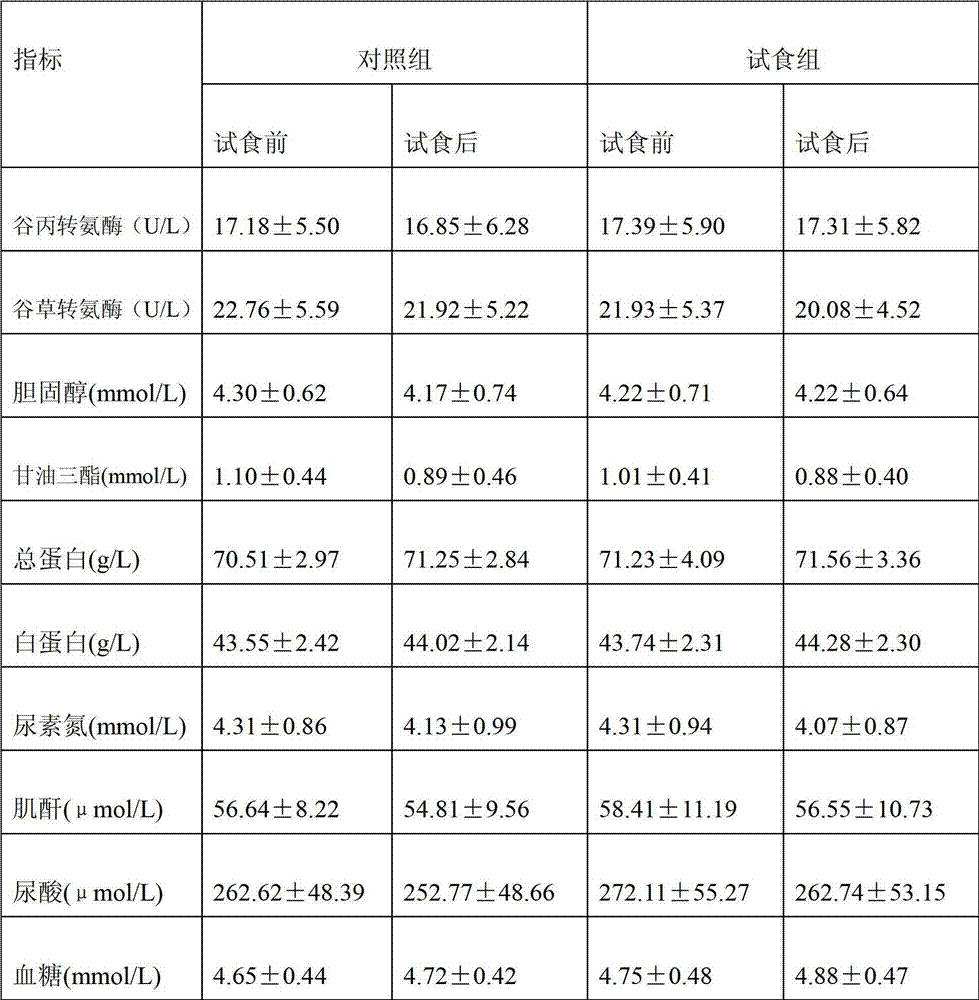
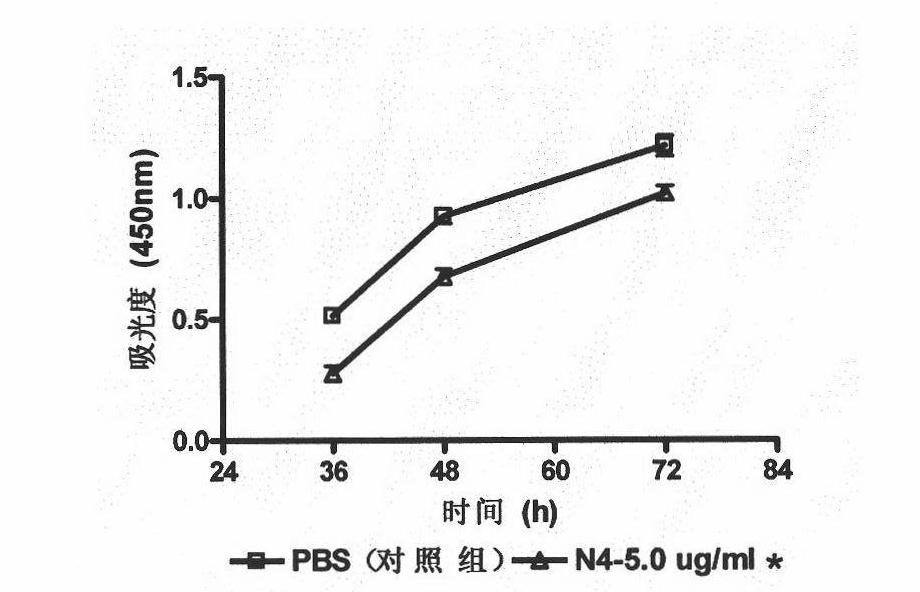
Patents
Literature
644 results about "Eye drops solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases
This invention provides a polymeric drug delivery system including a hydrogel containing one or more drugs for the treatment of a posterior segment disease. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Methods and articles for the delivery of medicaments to the eye for the treatment of posterior segment diseases
InactiveUS20050255144A1Pharmaceutical delivery mechanismEye treatmentHigh concentrationDelivery system
This invention provides articles and methods for drug delivery including a hydrogel containing one or more drugs for the treatment of a posterior segment disease and / or dry eye conditions. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Composition and method for healing tissues
InactiveUS20050147679A1Enhances chemotactic activityGood healing effectOrganic active ingredientsBiocideAdhesiveAdditive ingredient
The composition and method for healing tissues is a medicinal composition for facilitating the growth, protection and healing of tissues and cells in animals and humans. The composition is formulated as a either a powder, gel, paste, film, fluid injectable, rehydratable freeze-dried paste or sponge, sprayable solution, topically applied patch with adhesive and reservoir system, an intermediate for coatables such as films and bandages, a matrix for membranes, or as a matrix of flexible polymer(s), or delivered as either an orally ingestible liquid, tablet or capsule. The main ingredient of the formulated compositions is hydrolyzed collagen, which can be combined with polysulfated glycosaminoglycans, hyaluronic acid or salts thereof, or a glucosamine salt, and mixtures thereof. The composition may be formulated as an aqueous eye drop solution.
Owner:PETITO GEORGE D +1
Lubricant for the ocular surface
InactiveUS20120128763A1Easy to spreadStabilize the tear filmOrganic active ingredientsSenses disorderNatural productIrritation
Owner:MELBJ HLDG
Precision Lid Retracting Eyedropper Device
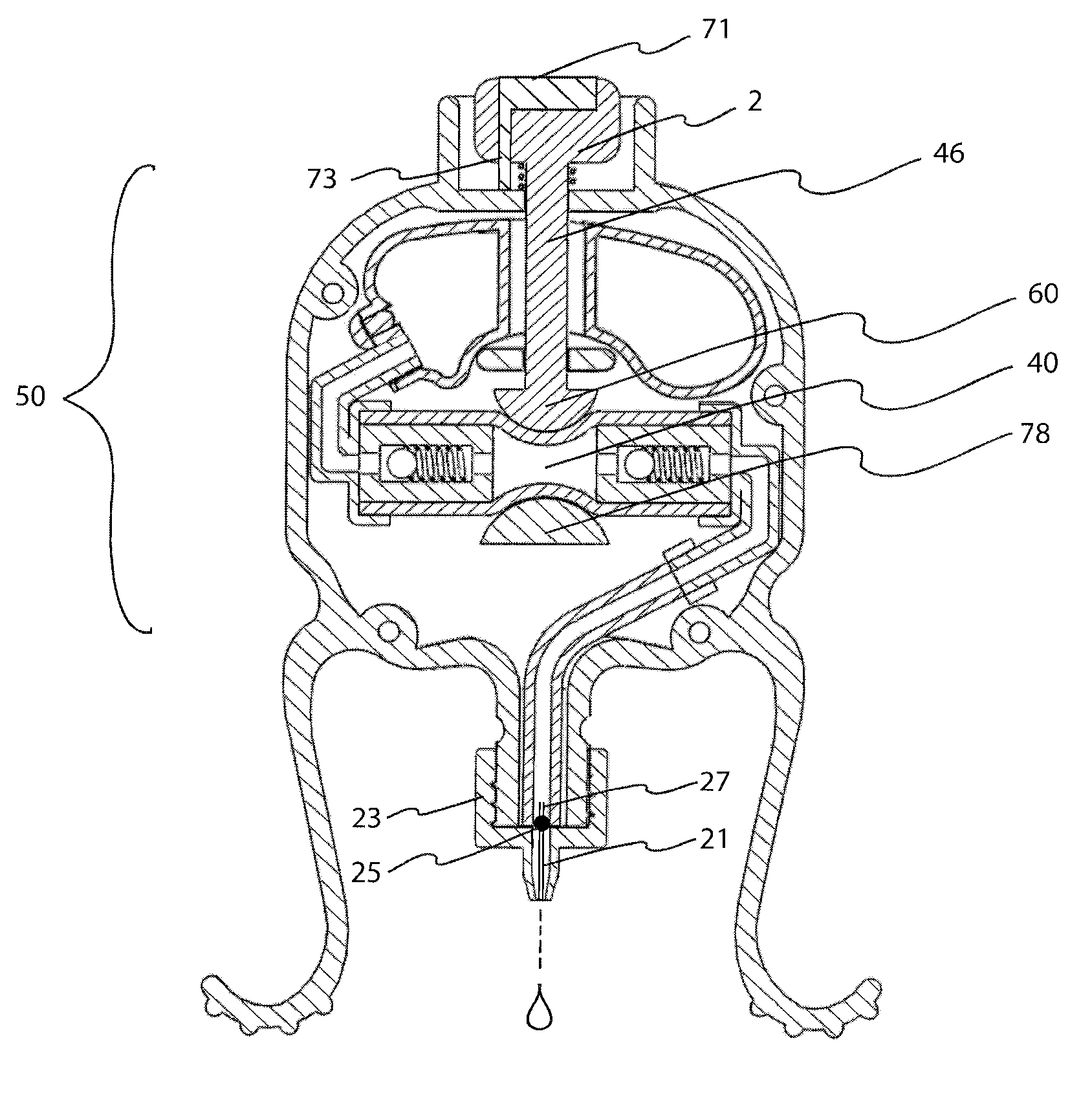
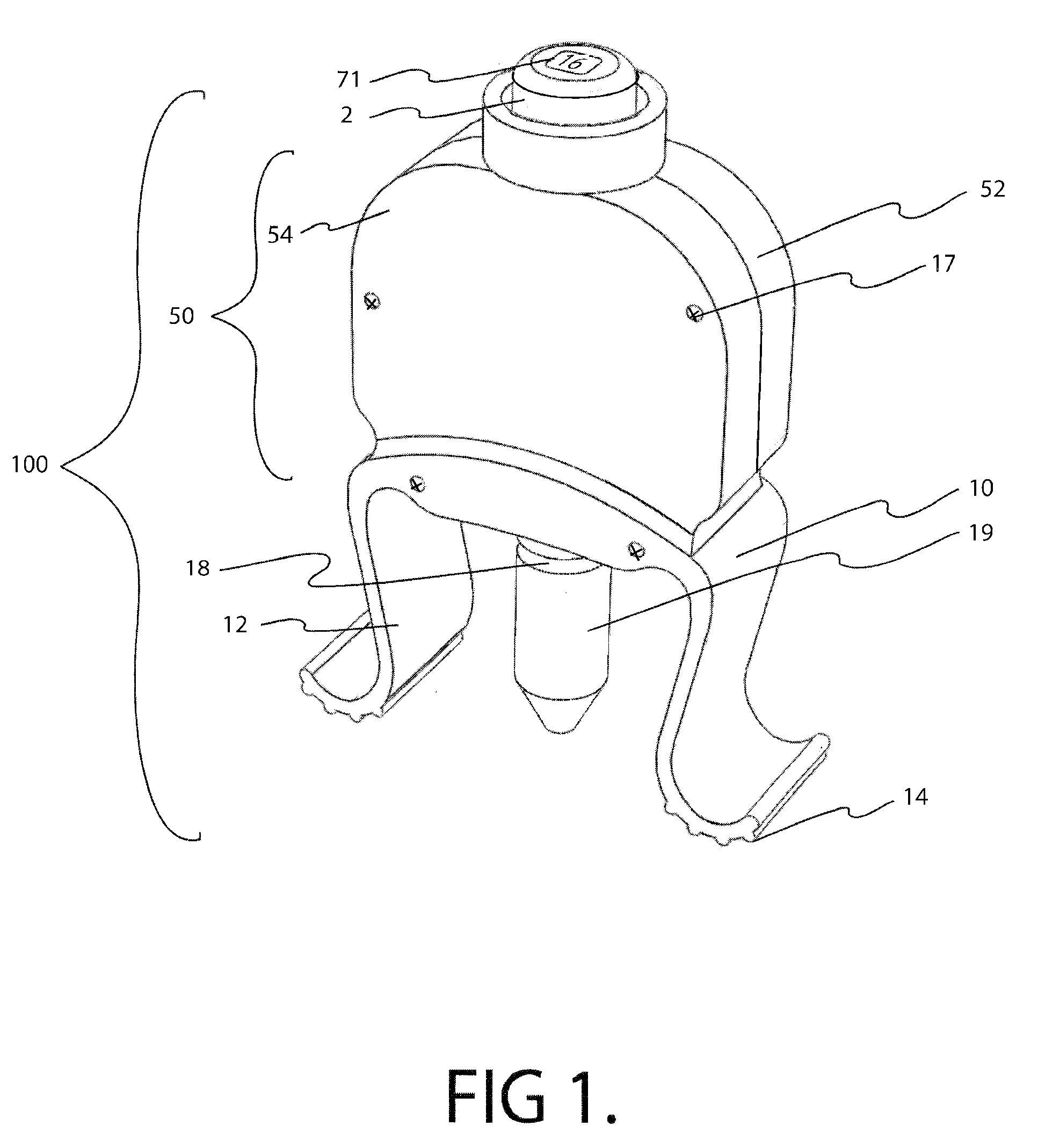
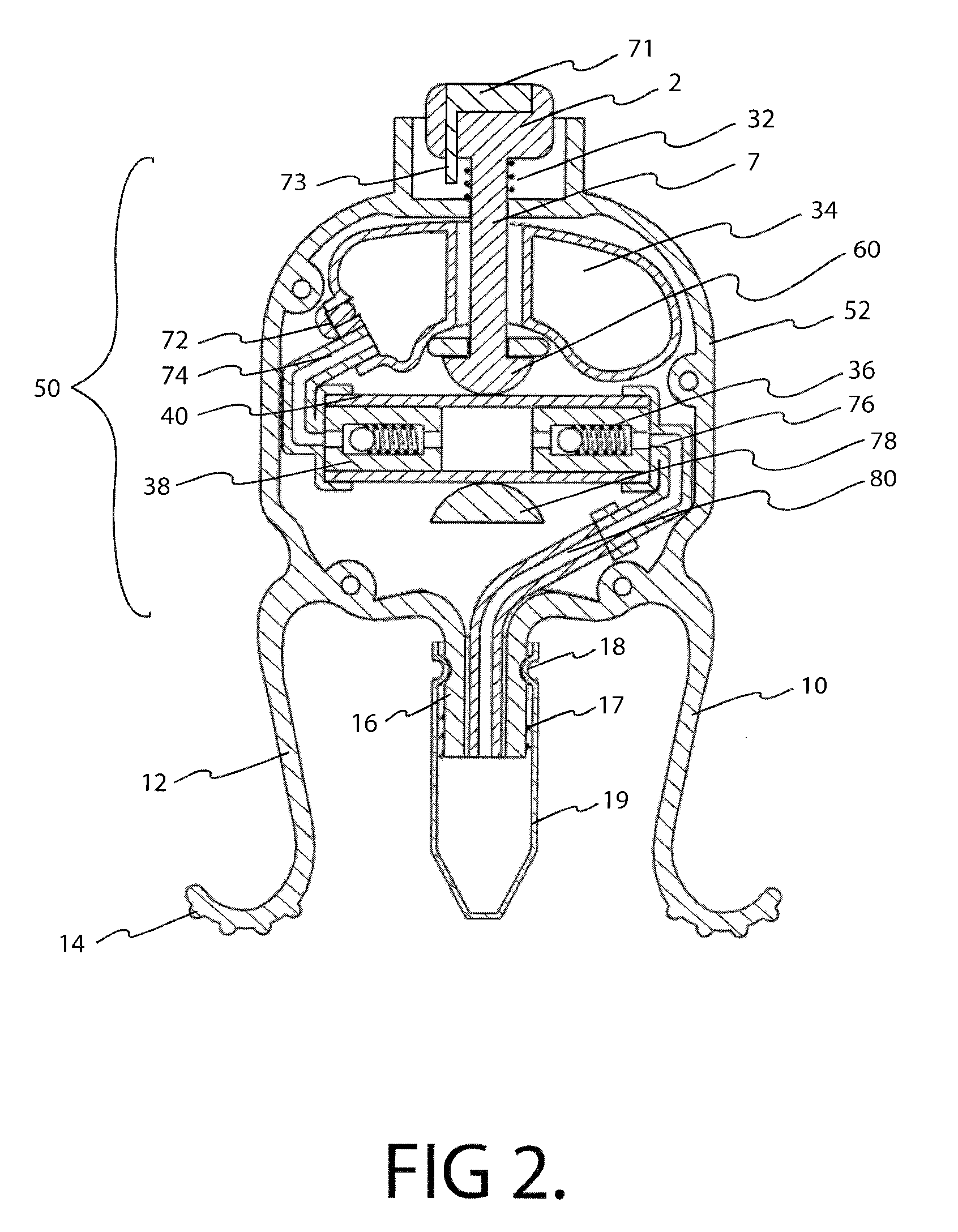
InactiveUS20100286633A1Precise and consistent amountEasy and economical to manufactureMedical applicatorsBathing devicesLid retractionEngineering
Precision lid retracting eyedropper device with a hollow, rigid housing having an upper aperture that allows a push button topped piston to enter the housing where the piston can impinge on a resilient tubular member that is part of a pump assembly. The housing also includes a solution storing chamber that feeds solution to the pump assembly. A solution exit tube attached to the outgoing portion of the pump assembly emanates from the bottom part of the housing and can be directed towards the user's eye. Integral downwardly facing resilient legs are placed just above and below the user's orbital ridge so that when the legs are squeezed and released, the user's eye lids are forced open allowing the user to dispense a precise amount of solution into his or her eye. A precision eye drop solution container having the ability to retain and dispense a precise single portion of eye drop solution each time the user depresses an actuator device located on and within the housing of the container. The container includes downwardly facing legs and feet that are parallel to each other and spaced so that the foot of each leg can align and make contact with the upper and lower orbital socket of the user. The feet have rubber-like under surfaces whose soft yet gripping quality acts to help hold the eye lids of the user in the open position. The dispensing action can be automated and an LED light indicating that dispensing has occurred.
Owner:MARX ALVIN J
Ophthalmic solution
An improved ophthalmic composition comprising an anionic polymeric substance, such as Hyaluronic Acid and / or Carboxymethylcellulose, in combination with any of various cationic monomeric or dimeric antimicrobial agents, such as Cetylpyridinium Chloride and / or Alexidine Dihydrochloride, wherein said compositions provide additional comfort and biocompatibility with lenses without significantly affecting the antimicrobial efficacy of the antimicrobial agent and without therefore requiring a substantially increased concentration of the agent such as that could expose contact lens wearers to increased levels of the disinfecting agent. Solutions according to the present invention may be used for effective multipurpose contact lens disinfection compositions, lens packaging solution compositions, and / or eye drops such as rewetters and tears.
Owner:ABBOTT MEDICAL OPTICS INC
Stabilized ocular solutions
InactiveUS20050065091A1Improve the ability to solveAntioxidant inhibitionBiocideSenses disorderVolume replacementAntioxidant
Ocular solutions containing an antioxidant provide beneficial properties, for example, the antioxidant scavenges free radicals in the solution which may cause the solution to deteriorate. However, antioxidants are themselves extremely susceptible to oxidation. A stabilizing agent for the antioxidant retards or prevents the antioxidant from undesirable reactions and thus enhances its ability to stabilize the ocular solution. This in turn enhances the physiological properties of the ocular solution, which may be a topical solution such as eye drops, or a surgical ocular irrigation or volume replacement solution.
Owner:MINU
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure(tm) from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Eye cyclosporin gel
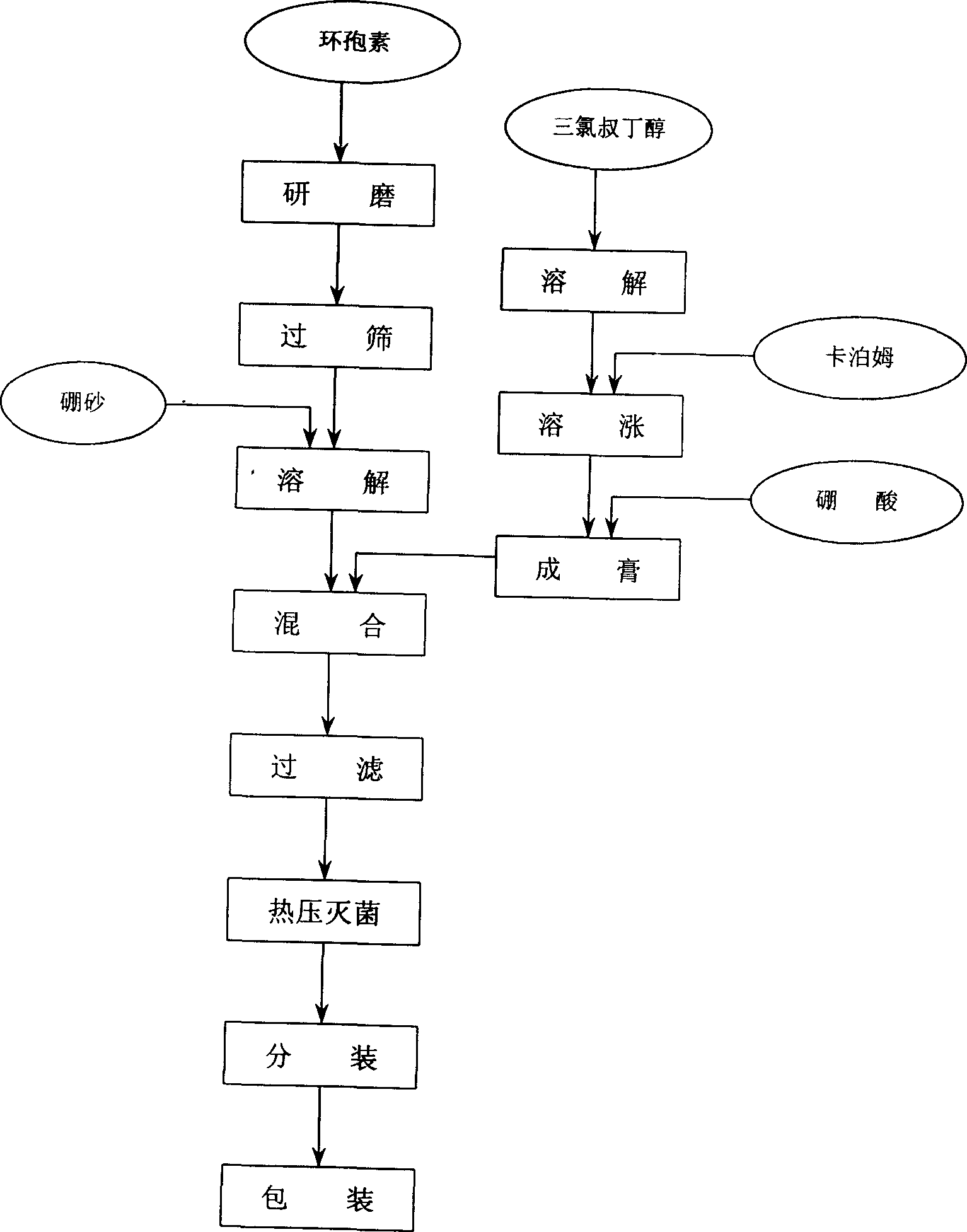
InactiveCN1456350ANot easy to diluteGood water solubilitySenses disorderPharmaceutical delivery mechanismCyclosporinsWhole body
A cyclosporin eye gel for treating the rejection reaction of corneal transplantation, keratoconjunctival xerosis, catarrhal conjunctivitis, and chemical burn of eye is prepared from cyclosporin, boric acid, borax, trichloro-tert-butanol, etc. Its advantages are long stay time in eye and high curative effect.
Owner:刘继东
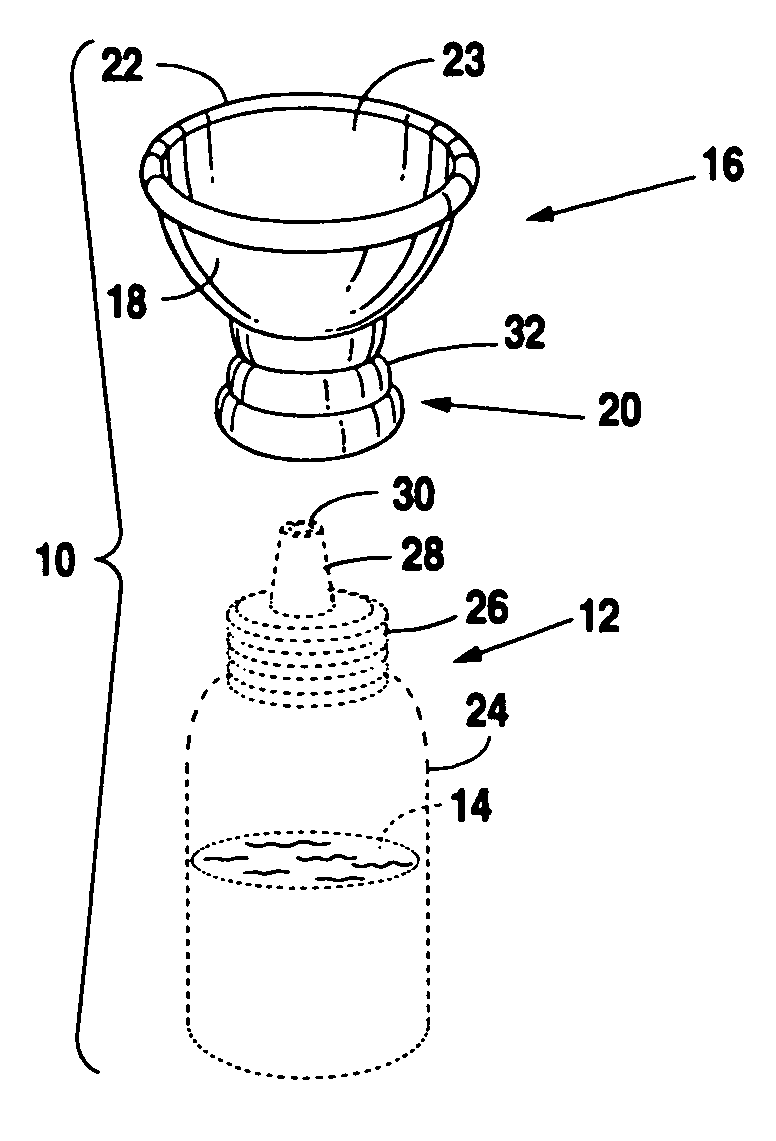
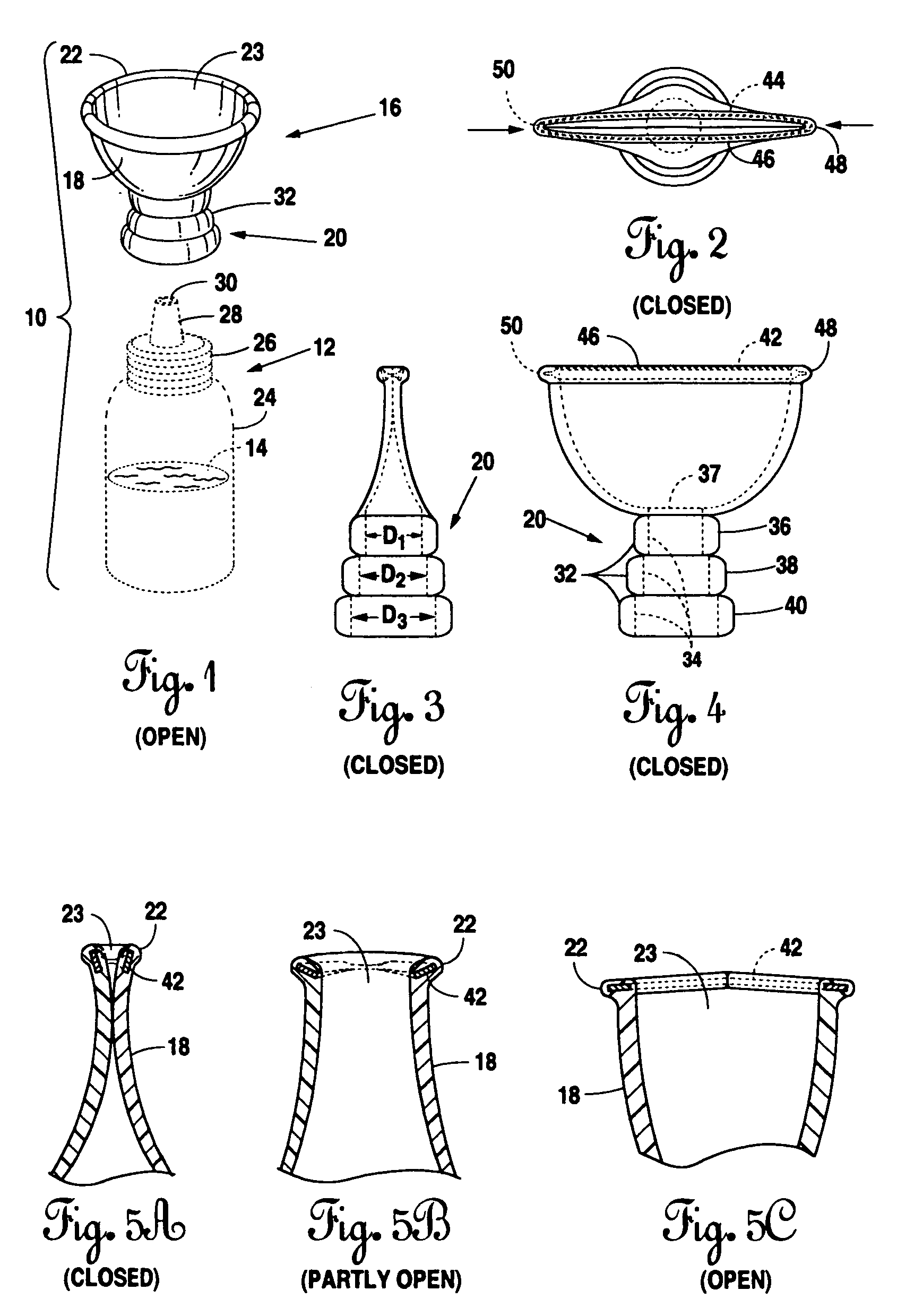
Eye drop applicator
InactiveUS20060129113A1Avoid pollutionEasy to manufactureMedical applicatorsEye treatmentEngineeringSpherical shaped
A novel applicator for engagement to an eye drop vial. The eye drop vial would be squeezable and contain a liquid therein that is compatible with the eye. The adapter includes a neck having a multiplicity of inner diameters adapted to a multiplicity of eye drop vials and a cup. The cup is semi-spherical shaped and has a rim that is collapsible between a closed position, which would seal off the vial, and an open position which would allow application of liquid from the vial to the eye of a user.
Owner:MERRICK JAMES
Voriconazole eye drops and preparation method thereof
InactiveCN101849905AImprove comfortGood for antifungal effectOrganic active ingredientsSenses disorderMedicineBromine
The invention discloses voriconazole eye drops. Every 1000 milliliters of eye drops contain 3-50g of voriconazole powder, 20-500g of hydroxypropyl-beta-cyclodextrin, 0.1-0.3g of bacteriostat, 0.5-2.5g of sodium hyaluronate and the balance of water for injection, wherein the bacteriostat is benzalkonium chloride, benzalkonium bromine or ethylparaben. The invention also discloses a preparation method of the voriconazole eye drops. The invention greatly increases the solubility of voriconazole in water to 0.3-5%, so that the medicine can exist in water solution in the form of molecules, thereby providing convenience for exerting the antifungal action of the medicine. Meanwhile, when being applied to eyes, the invention provides convenience for exerting the antifungal action of the medicine through cornea. The preparation method can be carried out at room temperature without affecting the medicine stability, and has the advantages of simple and easy realization and low cost.
Owner:河南省眼科研究所
Transparent eye drops containing latanoprost
InactiveUS20060069162A1White turbidity can be preventedAvoid turbidityBiocideSenses disorderPreservativeMedicine
An object of the present invention is to provide better formulations of a latanoprost ophthalmic solution. The present invention provides a clear ophthalmic solution comprising latanoprost as an active ingredient and benzalkonium chloride as a preservative wherein white turbidity due to a change of formulation is prevented by at least one means selected from the following 1) to 3); 1) adding a surfactant, 2) using benzalkonium chloride represented by the formula of [C6H5CH2N(CH3)2R]Cl (wherein R is alkyl having 12 carbon atoms) as the preservative and 3) adding a nonionic tonicity agent as a tonicity agent.
Owner:SANTEN PHARMA CO LTD
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure™ from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Use of a VEGF Antagonist in Treating Retinopathy of Prematurity
InactiveUS20160159893A1Stopping abnormal blood vessel growthExtended half-lifeLaser surgerySenses disorderDiseaseRetina
The present invention relates to the use of a VEGF antagonist in the treatment of retinal neovascular disorders in infants. In particular, the invention provides a method for treating an infant having retinopathy of prematurity (ROP), wherein said method comprises administering to the eye of an infant a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The term “infant” is typically used to refer to young children from birth up to the age of 12 months. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops.
Owner:NOVARTIS AG
Liquid eye drop composition
A composition that is used as an eye treatment contains reduced glutathione, vitamin A and vitamin E, as well as one or more of zinc sulfate, boric acid and potassium as buffering agents. The composition also may contain a lubricant and a preservative. The composition is a sterile isotonic solution. The composition is used in a method of treating eyes for the alleviation of irritations and / or dryness, as well as for the prevention and treatment of cataracts.
Owner:BRASWELL A GLENN +2
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20070104744A1Discomfort to userDegree of reductionOrganic detergent compounding agentsLens cleaning compositionsOphthalmic solutionsBiology
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Compounding use of sodium hyaluronate for eye preparation
InactiveCN1488404AImprove stabilityImprove water holding capacitySenses disorderPharmaceutical non-active ingredientsGlycerolWater soluble
The invention refers to the compound applied technique of two components of adjunct medical material-sodium hyaluronate and glycerin, in ophthalmic preparation. Its main technique: the compound base material is added to the ophthalmic preparation to make into stable water-soluble transparent eye drops; sodium hyaluronate is 0.05%-0.5% (g / ml) and glycerin 0.1%-2.5% (g / ml), and the mixed proportion: the former : the latter=1:1-1:4, and the optimal project: the former 0.05%-0.25%, the latter 0.1%-0.5%, and the mixed proportion: 1:2-1:4, and they can be added in multiple eye drops.
Owner:刘继东
Preservative Removal from Eye Drops
ActiveUS20170224531A1Reduce or eliminate further drug uptakeOrganic active ingredientsSemi-permeable membranesPreservativeMicroparticle
A BAK removal device is constructed as a plug of microparticles of a hydrophilic polymeric gel that displays a hydraulic permeability greater than 0.01 Da. The polymer hydrophilic polymeric gel comprises poly(2-hydroxyethyl methacrylate) (pHEMA). The particles are 2 to 100 μm and the plug has a surface area of 30 mm2 to 2 mm2 and a length of 2 mm to 25 mm and wherein the microparticles of a hydrophilic polymeric gel has a pore radius of 3 to 60 μm.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Eye Drops for the Treatment of Dry Eye
InactiveUS20070265353A1Eliminate the effects ofSuppression problemBiocideSenses disorderSqualaneCompound (substance)
Eye drops for the treatment of dry eye is disclosed. The eye drops is a solution-type, emulsion-type or two separate phase-type eye drops containing squalane and is, among others, an oil-in-water-type emulsion further containing a water-soluble macromolecular compound such as polyvinylpyrrolidone. Also disclosed are use of squalane for the production of eye drops for the treatment of dry eye, and a method for the treatment of dry eye.
Owner:SENJU PHARMA CO LTD
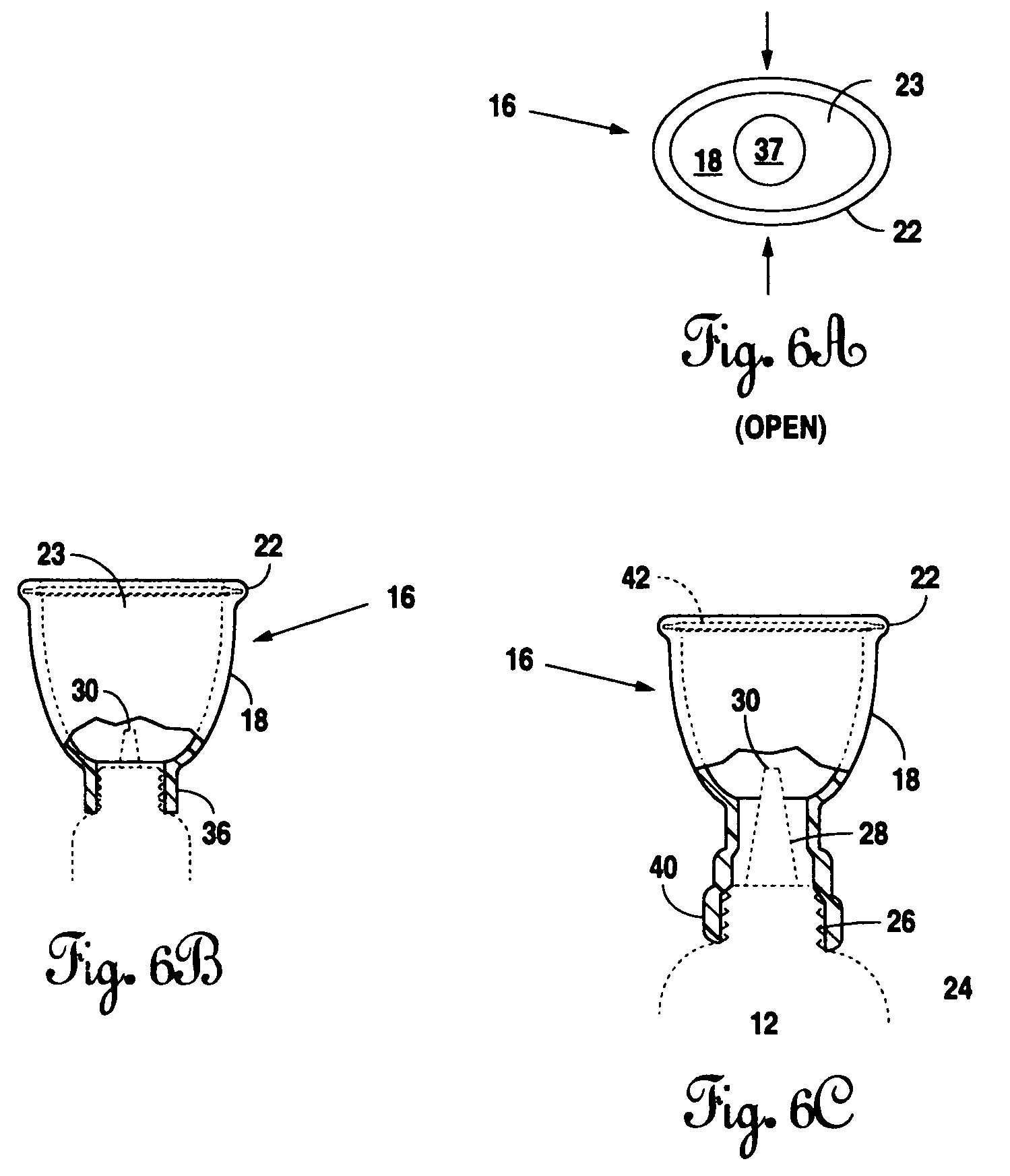
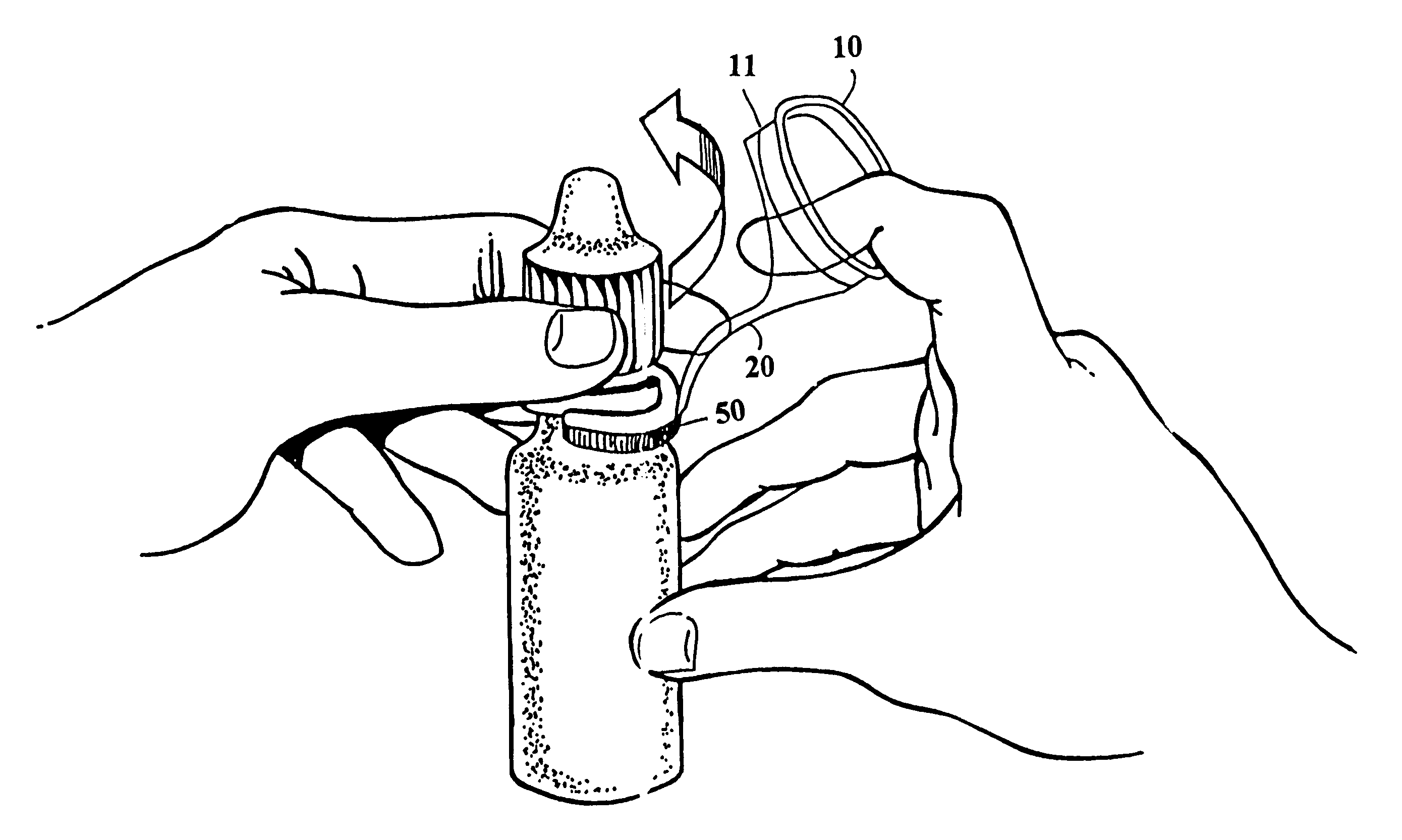
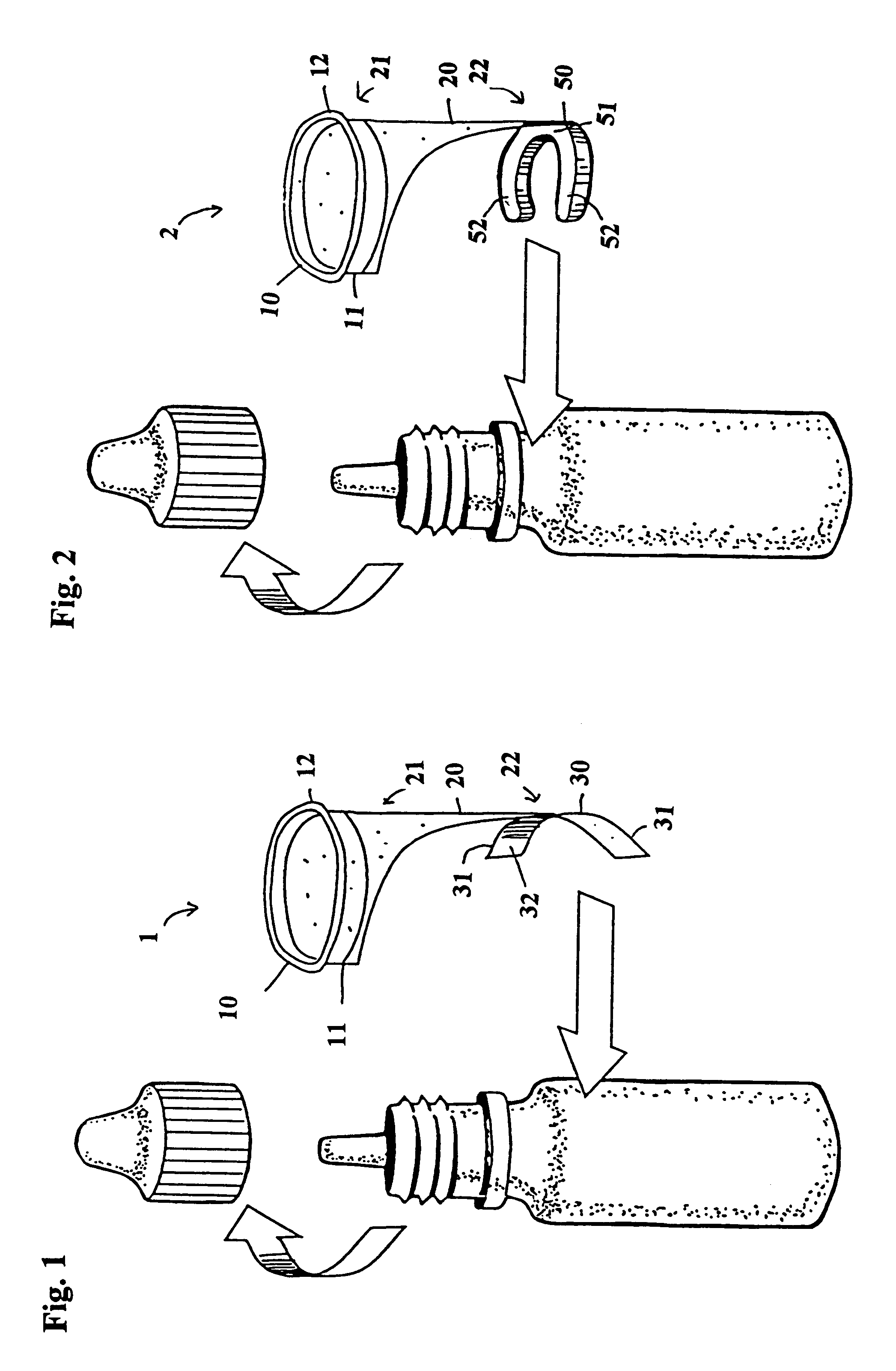
Eye dropper positioning device
InactiveUS6530908B1Easy to removeEasy to replaceMedical applicatorsBathing devicesBottle capEye drop
The present invention is directed to a device to aid in the placement of eye drops from an ophthalmic medication containing bottle. The device of the present invention is designed to be attached to the bottle at all times, including while in use. Furthermore, the present invention is specifically designed to aid in the easy removal and replacement of ophthalmic solution containing bottle caps. The present invention, in its most basic form, comprises an eye ring, a bottle attaching portion and a flexible extension connecting the eye ring to the bottle attaching portion, wherein the eye ring and flexible extension may be moved off of the bottle axis to permit easy removal and replacement of the bottle cap.
Owner:SHERMAN THOMAS M +1
A kind of azithromycin gel eye drop and its preparation process
ActiveCN102283799AStrong practical significanceStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAntioxidant
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
Eye drops containing chitosan derivative
InactiveCN1403158AImprove bioavailabilityGood curative effectSenses disorderPharmaceutical non-active ingredientsClinical efficacyAdditive ingredient
The present invention relates to medicine technology, and is one kind of eye drops for treating conjunctivitis, keraritis, corneal ulcer, xerophthalma and other eye diseases. The eye drops contains water soluble chitosan derivative and medicinal active component, proper amount of pH controlling agent and osmotic pressure regulator. It has delayed releasing and long acting function and thus high biological medicine utilization and clinical curative effect.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Stable eye drops containing latanoprost as the active ingredient
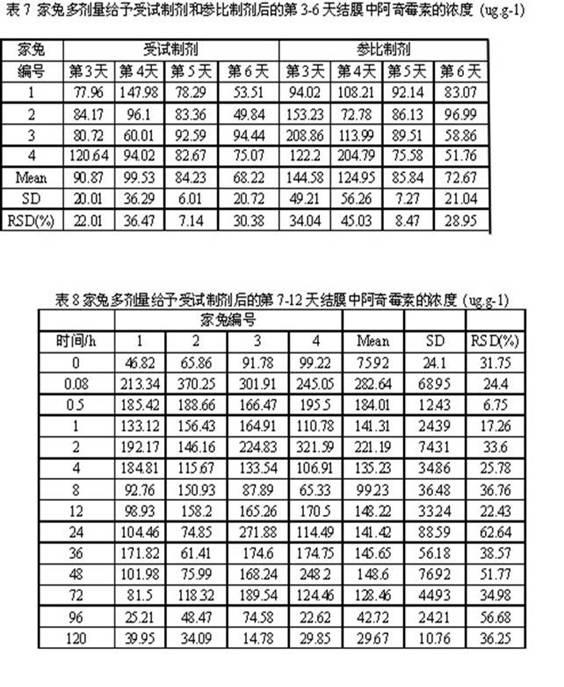
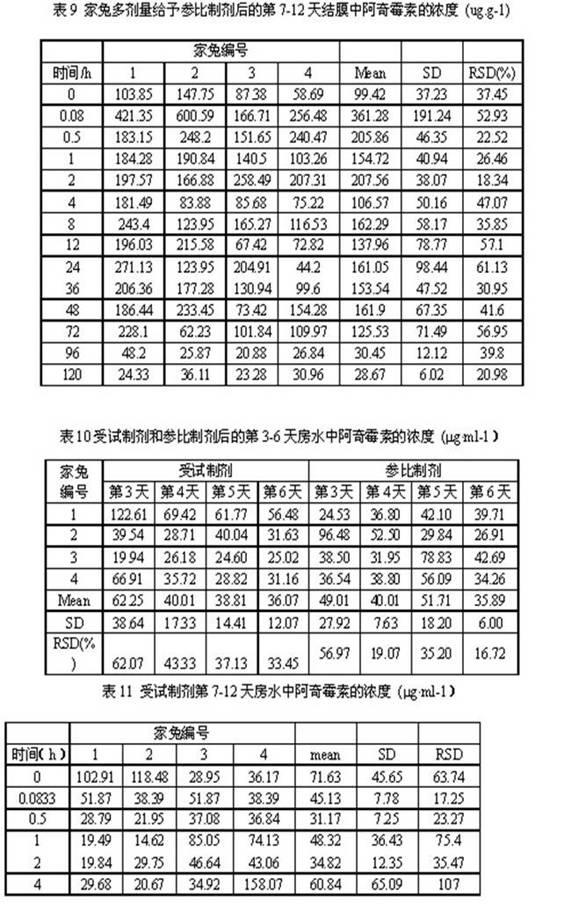
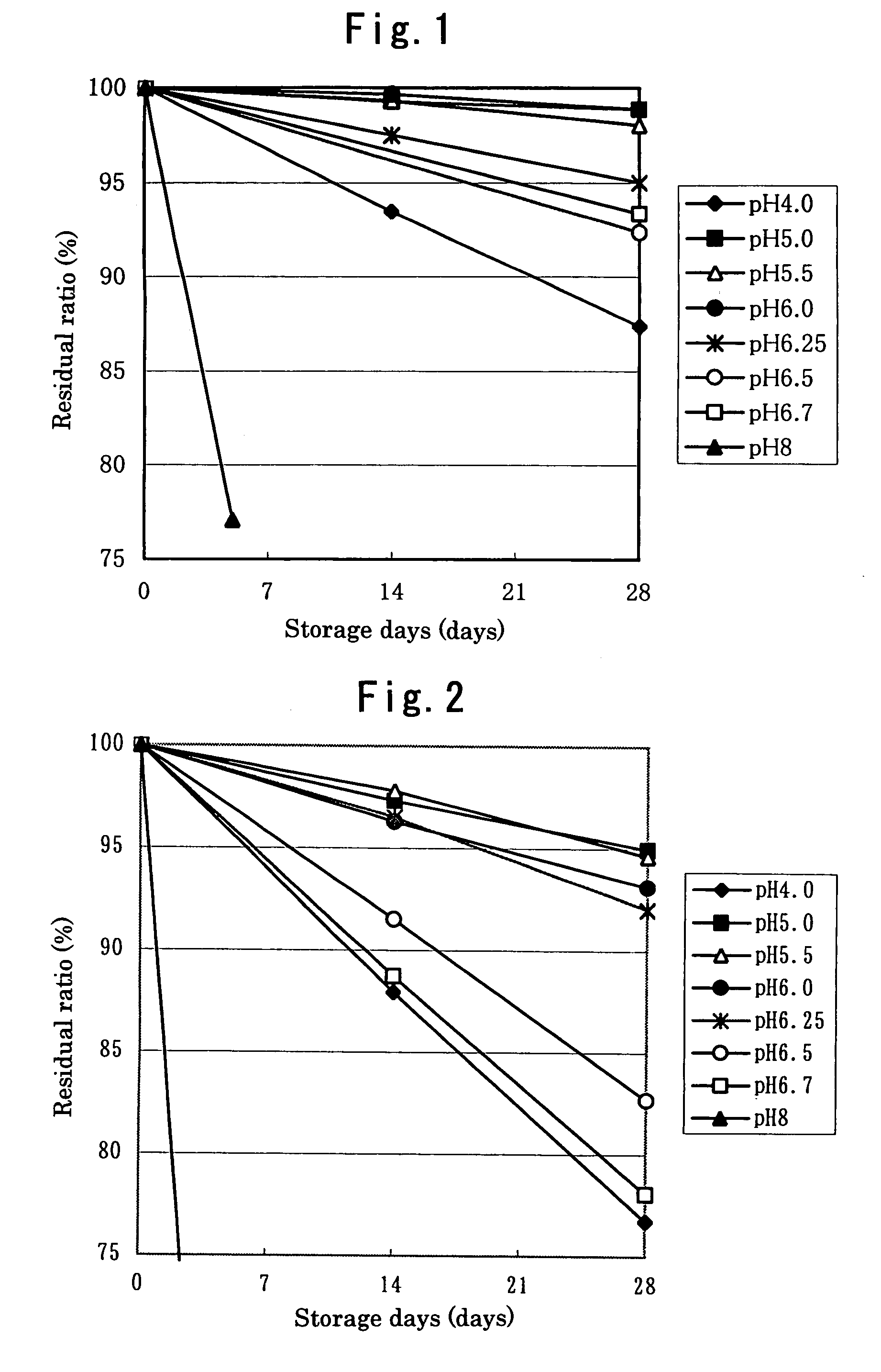
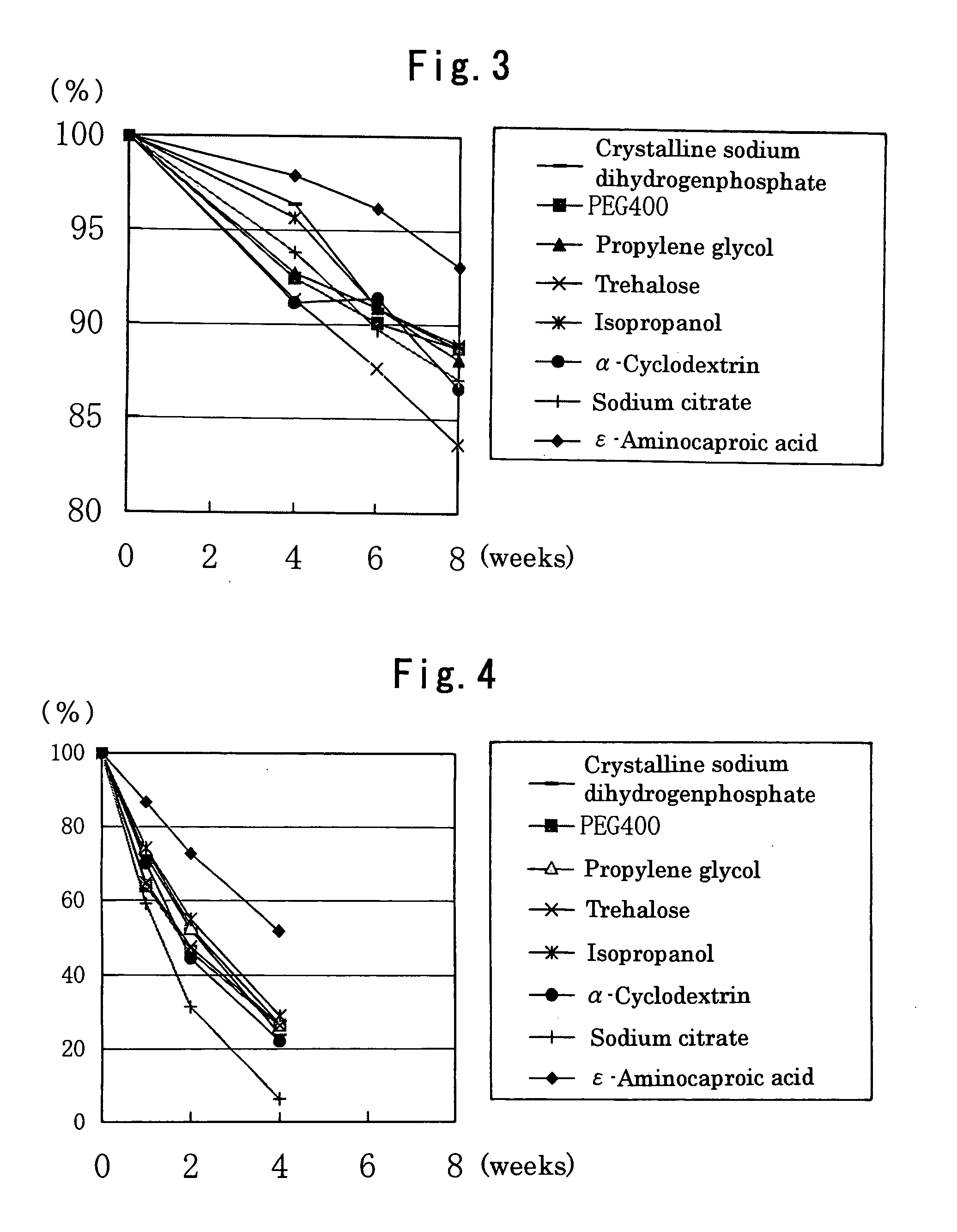
The present invention provides a latanoprost ophthalmic solution which can be stored at room temperature and is excellent in stability. The ophthalmic solution according to the present invention is an ophthalmic solution comprising latanoprost, wherein latanoprost is stabilized to be stored at room temperature by at least one means selected from the following 1) and 2); 1) adjusting pH of the solution to 5.0 to 6.25 and 2) adding ε-aminocaproic acid to the solution.
Owner:SANTEN PHARMA CO LTD
A kind of preparation method of non-ionic cellulose eye drops
ActiveCN102283805ADissolve fastAvoid the risk of adverse sterility assuranceOrganic active ingredientsSenses disorderNon ionicThermal water
The invention discloses a method for preparing eye drops containing non-ionic cellulose derivatives, which is suitable for laboratory preparation and mass industrial production. By the preparation method, the problems that the non-ionic cellulose derivatives are difficult to disperse and dissolve in cold water and have overlong swelling consumed time in the cold water after being dispersed in hotwater can be effectively solved; and by the method, the non-ionic cellulose derivatives are quickly and effectively dissolved, and the eye drops containing the non-ionic cellulose derivatives are efficiently prepared in laboratories or in an industrialization way.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Composition and method for healing tissues
InactiveUS7691829B2Rapid hydrolysisChemical binding is enhanced chemotacticallyBiocideOrganic active ingredientsAdditive ingredientAdhesive
Owner:PETITO GEORGE D +1
Bendazac lysine eye drops with reduced irritability, its preparation method and application
InactiveCN1762350AIncrease irritationReduced response time to stimuliOrganic active ingredientsSenses disorderIrritationBuffering agent
The invention discloses a bendazac lysine eye drops with reduced irritability, its preparation method and application, wherein the eye drop is prepared from bendazac lysine, thickening agent, buffering agent, preservative agent, isotonic agent, substance for reducing eye drop pungency and water for injection.
Owner:浙江平湖莎普爱思制药有限公司
Bilberry and lutein soft capsule and preparation method thereof
InactiveCN102960738AFast onsetEffect does not bounceFood preparationLingonberry extractBeta-Carotene
The invention relates to a bilberry and lutein soft capsule, which aims to overcome the defects that a number of oral medicaments and eye drops taking lutein as a main ingredient for easing visual fatigue on the market are difficult to bring the efficacy of the lutein into full play due to unreasonable compatibility and can not fulfill the effect of easing the visual fatigue. The bilberry and lutein soft capsule comprises contents and glue liquor, wherein the contents are prepared from the following auxiliary materials in parts by weight: 70-88 parts of bilberry extracts, 36-45 parts of lutein ester, 8-12 parts of natural beta-carotene oil, 0.9-1.2 part(s) of natural vitamin E, 23-28 parts of beeswax and 320-380 parts of soybean oil. The invention further relates to a preparation method of the bilberry and lutein soft capsule. The bilberry and lutein soft capsule disclosed by the invention can bring the effects of the lutein into full play, becomes effective at a high speed, does not bounce in effect, has the advantages of being capable of consolidating curative effects, lasting in action and the like, and is widely suitable for treatment on functional visual fatigue and symptomatic visual fatigue, and in particular suitable for patients with the visual fatigue caused by long-term overuse of the eyes.
Owner:BY HEALTH CO LTD
Histatin for Corneal Wound Healing and Ocular Surface Disease
InactiveUS20130310326A1Promote wound healingPromote healingSenses disorderPeptide/protein ingredientsCorneal woundOcular surface disease
Histatins may be used for corneal wound healing and as a treatment for ocular surface disease in humans and other animals. For example, histatins could be included in eye drops, eye gels, ointment, glue, or embedded in (polymer) contact lenses.
Owner:VISUS THERAPEUTICS INC
Eye drop and preparation method thereof
ActiveCN101829048ASimple ingredientsReliable ingredientsSenses disorderPeptide/protein ingredientsLymphatic vesselFiltration
The invention discloses an eye drop and a preparation method thereof, relating to an eye drop. The invention provides an eye drop with short action time, high purity and relatively good effect and capability of simultaneously playing a role of corneal neovascularization, lymphangiogenesis and cornea inflammation resistance and a preparation method thereof. The eye drop contains the following components by volume percent: 0.1-5% of bovine serum, 5-15% of thickening agent, 1-5% of acid-base regulating solution, 0.5-2% of antibiotics, 5-20% of recombinant protein and the balance of balanced salt solution. The eye drop contains the balanced salt solution, the thickening agent, the bovine serum, the antibiotics, the acid-base regulating solution and the recombinant protein. The preparation method of the eye drop comprises the following steps of: adding the thickening agent, the bovine serum, the antibiotics and the recombinant protein to the balanced salt solution, regulating a pH value by using the acid-base regulating solution and osmotic pressure by using an osmotic pressure buffering agent, and removing bacteria through membrane filtration; or preparing the bovine serum to sterile micropowder, dissolving the bovine serum into the balanced salt solution, regulating the pH value, removing the bacteria through the membrane filtration, and dissolving recombined powder into the solution so as to obtain the eye drop.
Owner:XIAMEN UNIV
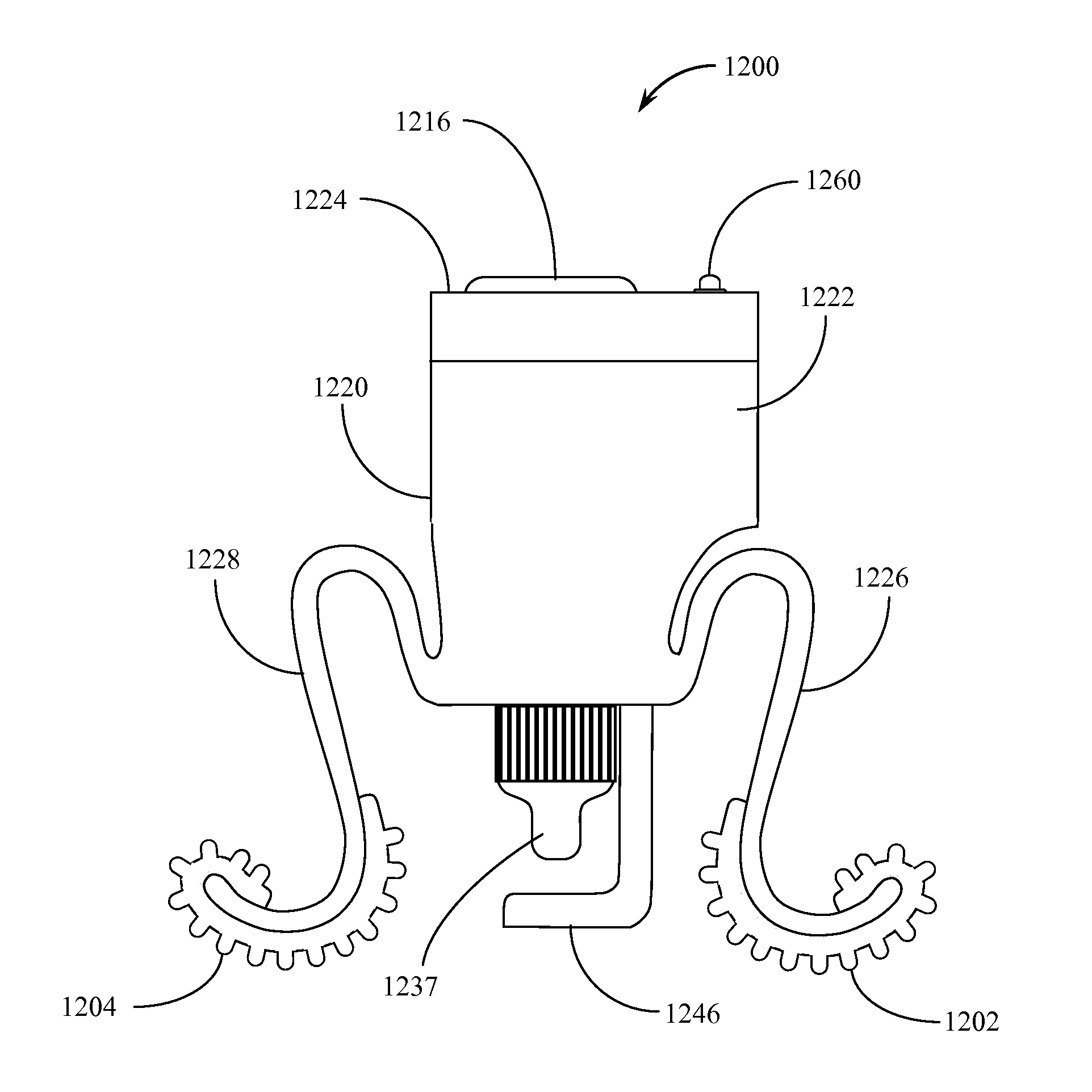
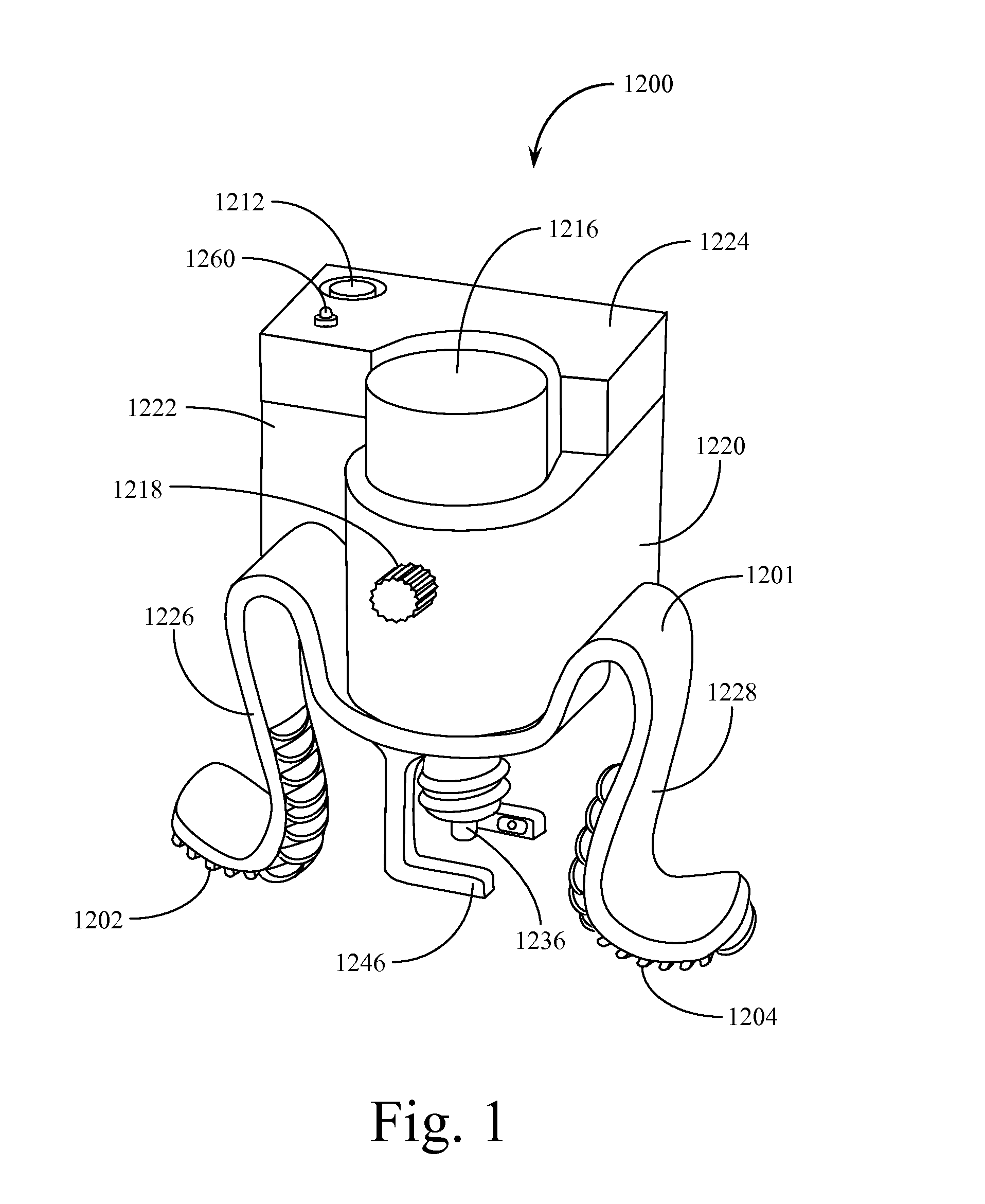
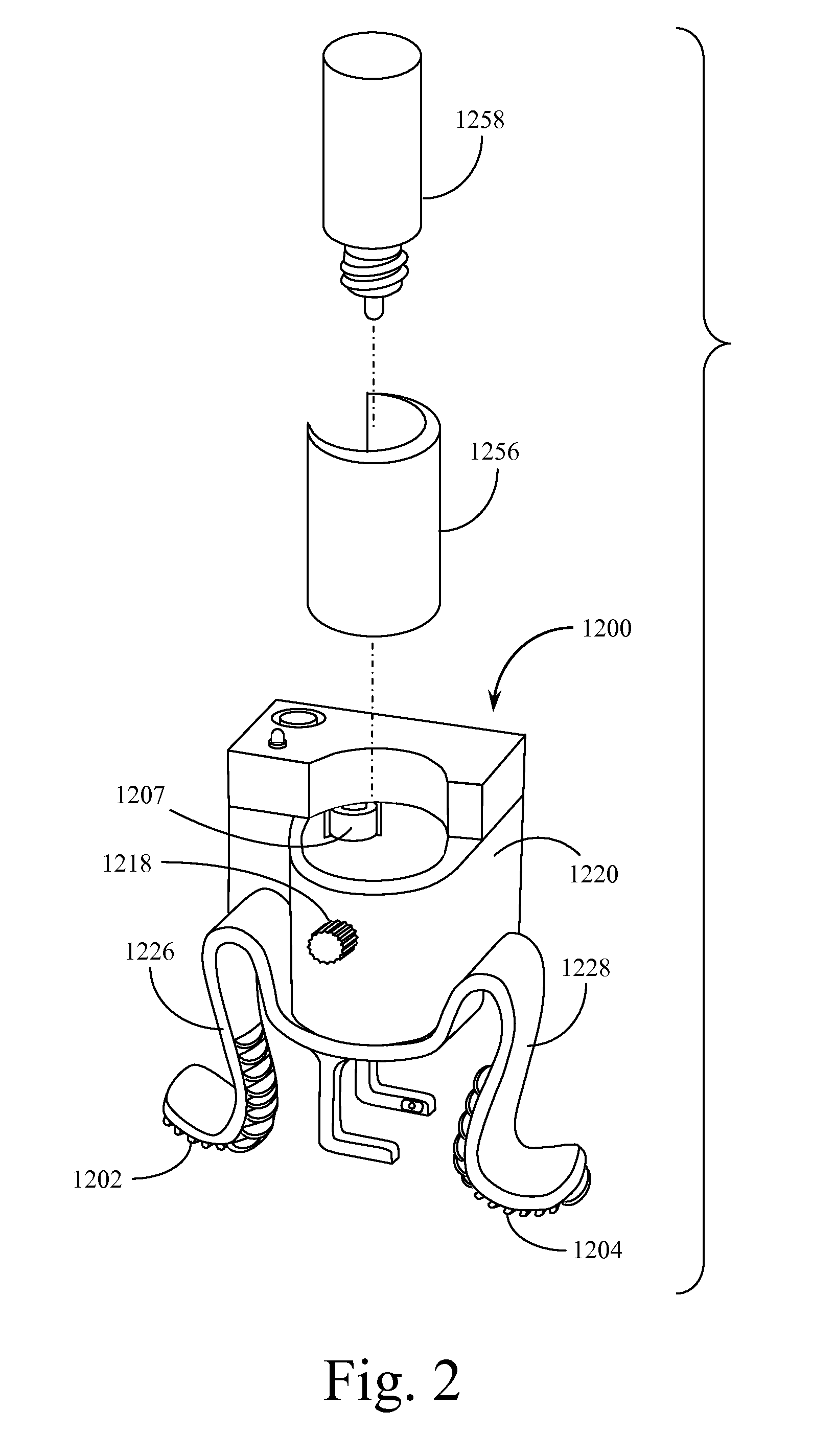
Automated Incremental Eyedrop Delivery System with Eyelid Retracting Legs
ActiveUS20130006202A1Facilitates proper positioningGood adhesionMedical applicatorsEye treatmentBottleCam
An eyedrop bottle holder with resilient arms formed from an inverted U-shaped band made from resilient injection molded plastic. The top middle portion of the inverted U-shape band includes a centrally located opening through which the top (dispensing portion) of a standard eyedrop bottle containing eyedrop solution may be positioned and held. The right and left arms of the U-shape band each terminate in an outwardly disposed J-shape foot, the underside of which is covered by a soft rubber-like pad. When a user inserts a standard eyedrop bottle into the holder, the dispensing tip of the eyedrop bottle may be positioned in close proximity to the user's eye. The user may cause his or her eye lid to remain open by using the fingers of one hand to squeeze the right and left arms of the holder together, then placing the pad of one arm on the upper ridge of the orbital eye socket and the pad of the second arm on the lower ridge of the orbital eye socket. Releasing the arms causes the skin of the user's upper and lower eyelids to be spread apart from each other and remain spread during an eyedrop solution dispensing event. The eyedrop bottle is retained within a housing that includes an electromechanical assembly with a rotating cam that pushes on the side of the bottle until a predetermined amount of solution is dispensed. When a set amount of eyedrop solution has been dispensed, the assembly resets itself. An electronic sensor detects the passage of a drop of solution from the bottle and directs a reverse motion to the rotating cam. Tilt sensors assist the user in properly orienting the device for use. An adaptor may be used to accommodate non-standard sized eyedrop bottles.
Owner:MARX ALVIN J
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com