Tissue engineered cornea epithelial transplantation membrane and preparation method and use thereof
A technology of tissue engineering and corneal epithelial cells, applied in the field of bioengineering and clinical medicine, to achieve the effect of eliminating the original antigen, avoiding immune rejection, and good tissue compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
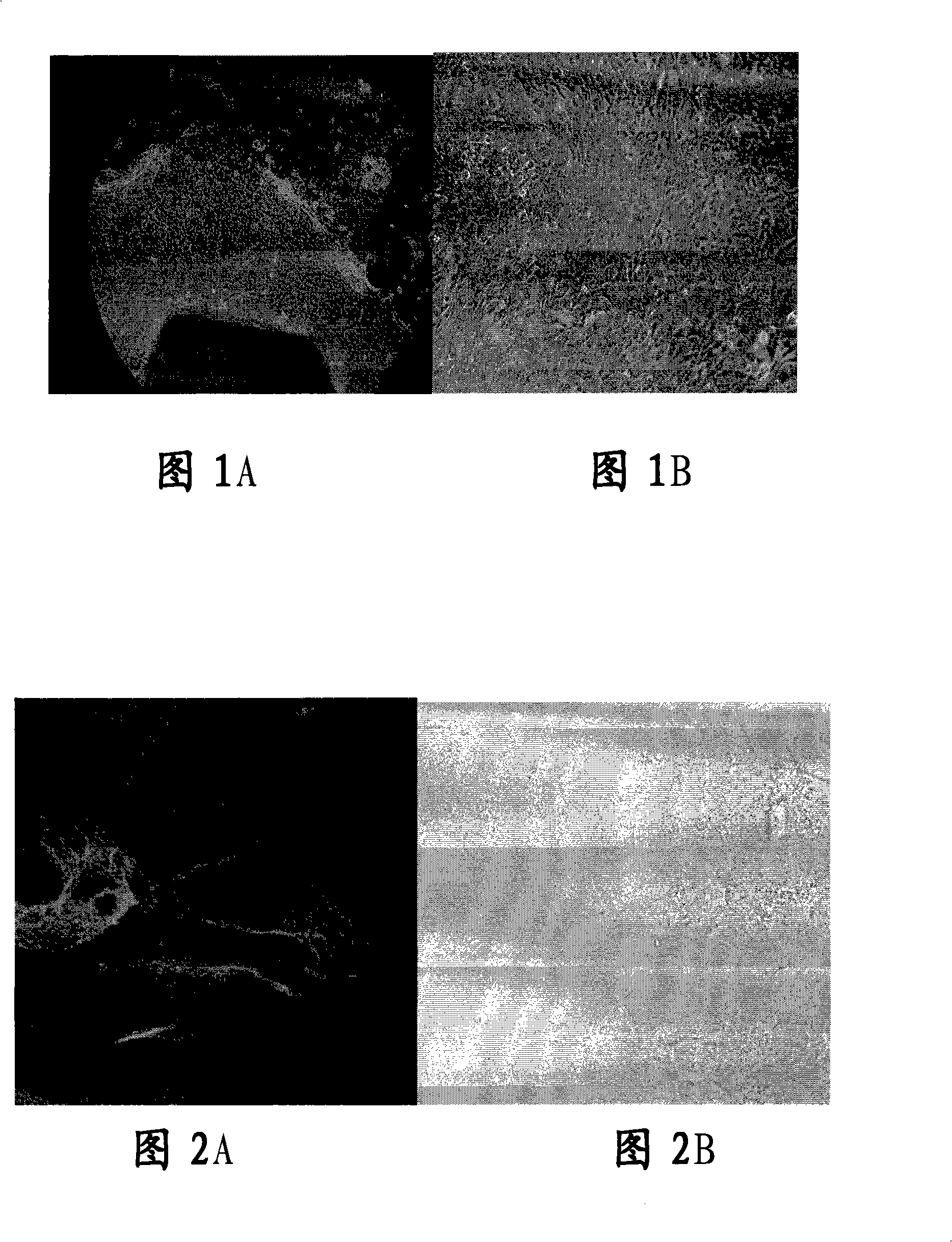
[0054] Embodiment 1, limbal tissue culture of human corneal epithelial cells in vitro
[0055] 1. In vitro primary and subculture corneal epithelial cells from corneal limbal tissue blocks
[0056] Primary culture: Disinfect human conjunctival sac and ocular surface with 10% povidone iodine, retrobulbar anesthesia with 2% lidocaine, use a cataract phacoemulsification incision knife (Alcon, U.S.A.) to cut a size of about 2.0 mm × 2.0 from the limbus of the human cornea The limbal tissue pieces with a thickness of about 200 μm were washed three times with D-PBS buffer containing 1% penicillin-streptomycin, then placed in 1.2U / ml dispase II digestion solution and kept overnight in the refrigerator at 4°C, after taking out - Wash with PBS buffer for 3 times, then treat with 0.25% trypsin-EDTA at 37°C for 10 minutes, and neutralize trypsin in DMEM culture medium containing 10% FBS. Place the limbal tissue pieces in a 60mm petri dish, dry naturally at room temperature in an ultra-c...
Embodiment 2
[0077] Embodiment 2. Tissue engineered corneal stent material
[0078] 1. Carrier selection:
[0079] There are currently many kinds of carriers for constructing tissue-engineered corneal epithelial transplantation membranes, among which amniotic membranes, collagen membranes and hydrophilic gel membranes are mostly used, but these membranes all have a common disadvantage, that is, they lack a certain thickness to resist tension, compression and refraction. Therefore, its application is limited. Heterogeneous acellular matrix, especially porcine acellular matrix, is easy to obtain and has no antigenicity after enzymatic digestion of porcine corneal stromal cells, and has the structural characteristics that cornea should have.
[0080] 2. Preparation of porcine acellular corneal stroma:
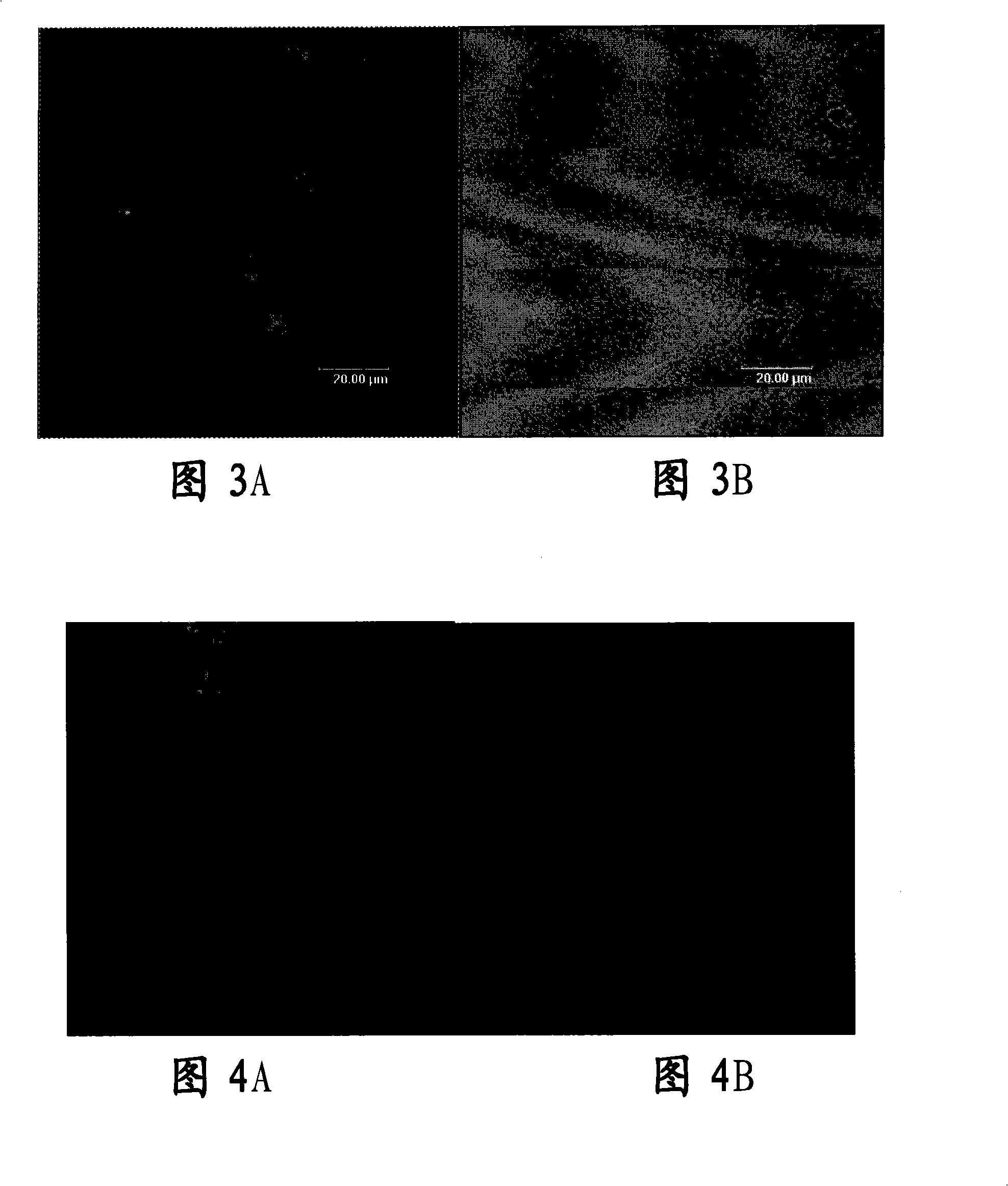
[0081] Such as Figure 14 As shown, fresh pig eyeballs were soaked in 0.125% chloramphenicol solution for 1 hour, soaked in 75% ethanol for 0.15 hours, scraped off the epithelium with a ra...
Embodiment 3
[0081] Such as Figure 14 As shown, fresh pig eyeballs were soaked in 0.125% chloramphenicol solution for 1 hour, soaked in 75% ethanol for 0.15 hours, scraped off the epithelium with a razor blade, cut the cornea along the corneal limbus, digested with 0.125% trypsin at 37°C for 30 minutes, PBS solution Shake and wash twice, immerse in a centrifuge tube of 1% TritonX2100 (or enter this step directly without enzymatic digestion), shake at 4°C for 72 hours. The PBS was shaken and washed repeatedly, and the sterile sealed package was flushed with D-PBS buffer containing 1% penicillin-streptomycin. Example 3: Preparation of Tissue-Engineered Corneal Epithelial Graft Membrane in Vitro
[0082] 1. Preparation of acellular porcine corneal stroma:
[0083] The corneal stroma prepared in Example 2 was soaked in fetal bovine serum for 24 hours and then placed in DMEM culture medium for future use.
[0084] 2. The corneal epithelial cells were subcultured in vitro and digested with 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com