Patents
Literature
32 results about "Cornea epithelium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1398644AGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
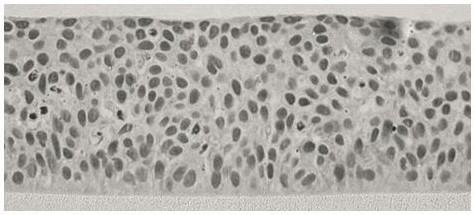
In the present invention an embryo or adult cornea epithelium is cut, digested with digesting liquid and centrifugated to prepare single cell suspension, and the cell suspension is cultured in culture dish or bottle with proper amount of culture medium and CO2 in 5% at 37 deg.c. The cultured cell is passed after cell converges to 80-90% and the second and sixth generation of cell is passed directly to amnion for culture for another 8-20 days to obtain the stem cell regenerating surface cornea of the present invention. The stem cell regenerating surface cornea may be used as material for treating corneal disease. The present invention provides a new material for treating corneal disease with rich material source, no danger of mouse-originated pollution, no or slight immunological rejection.
Owner:北京科宇联合干细胞生物技术有限公司
Cornea edge stem cell tissue engineering composite body and its preparation method
InactiveCN1590541APromote cell differentiationPromote differentiationArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsBiological tissueLimbal stem cell
A tissue-engineered complex of human limbus stem cell and amnion for treating the limbus stem cell-deficiency eye diseases is disclosed. It is perpared through removing epithelium from amnion, using it as carrier, using fibroblast 3T3 as nutritive layer, culturing by cell suspension method, and promoting differentiation of cells by air-lifting technique.
Owner:天津医科大学眼科中心
Cell sheets for ectocornea formation, method of producing the same and method of using the same
InactiveUS20060234377A1Prevent shrinkageEye implantsEye surgeryTemperature-responsive polymerCorneal epithelium
The present invention has as its objective providing acorneal epithelium forming cell sheet that will adhere well to an anterior segment tissue. To attain this objective, a corneal epithelium forming cell sheet is produced by a process comprising the steps of cultivating under specified conditions corneal epithelium forming cells on a cell culture support comprising a substrate having its surface covered with a temperature responsive polymer of which the hydrating force varies in a temperature range of 0-80° C., optionally stratifying the layer of cultured cells, and thereafter, (1) adjusting the temperature of the culture solution to either above an upper critical dissolution temperature or below a lower critical dissolution temperature, (2) bringing the cultured corneal epithelium forming cells into close contact with a carrier, and (3) detaching the sheet together with the carrier under specified conditions.
Owner:DELLSEED INC +2
Tissue engineered cornea epithelial transplantation membrane and preparation method and use thereof
InactiveCN101306207AMaintain biological characteristicsElasticEye implantsTransplanted corneaOphthalmology
The invention discloses a tissue engineering cornea epithelial transplantation membrane, a preparing method and an application thereof. The invention aims to provide a tissue engineering cornea epithelial transplantation membrane, the tissue engineering cornea epithelial transplantation membrane comprises a porcine cornea epithelial cell and nonantigenic tissue engineering cornea bracket material, wherein, the porcine cornea epithelial cell and the nonantigenic tissue engineering cornea bracket material are combined into a whole by adopting a tissue engineering method. And meanwhile, The invention further provides a method for preparing the tissue engineering cornea epithelial transplantation membrane, and a use of the tissue engineering cornea epithelial transplantation membrane during the process of preparing medical treatment material used for remedying blind eye disease caused by the pathological state and the damage of corneas.
Owner:PEKING UNIV THIRD HOSPITAL
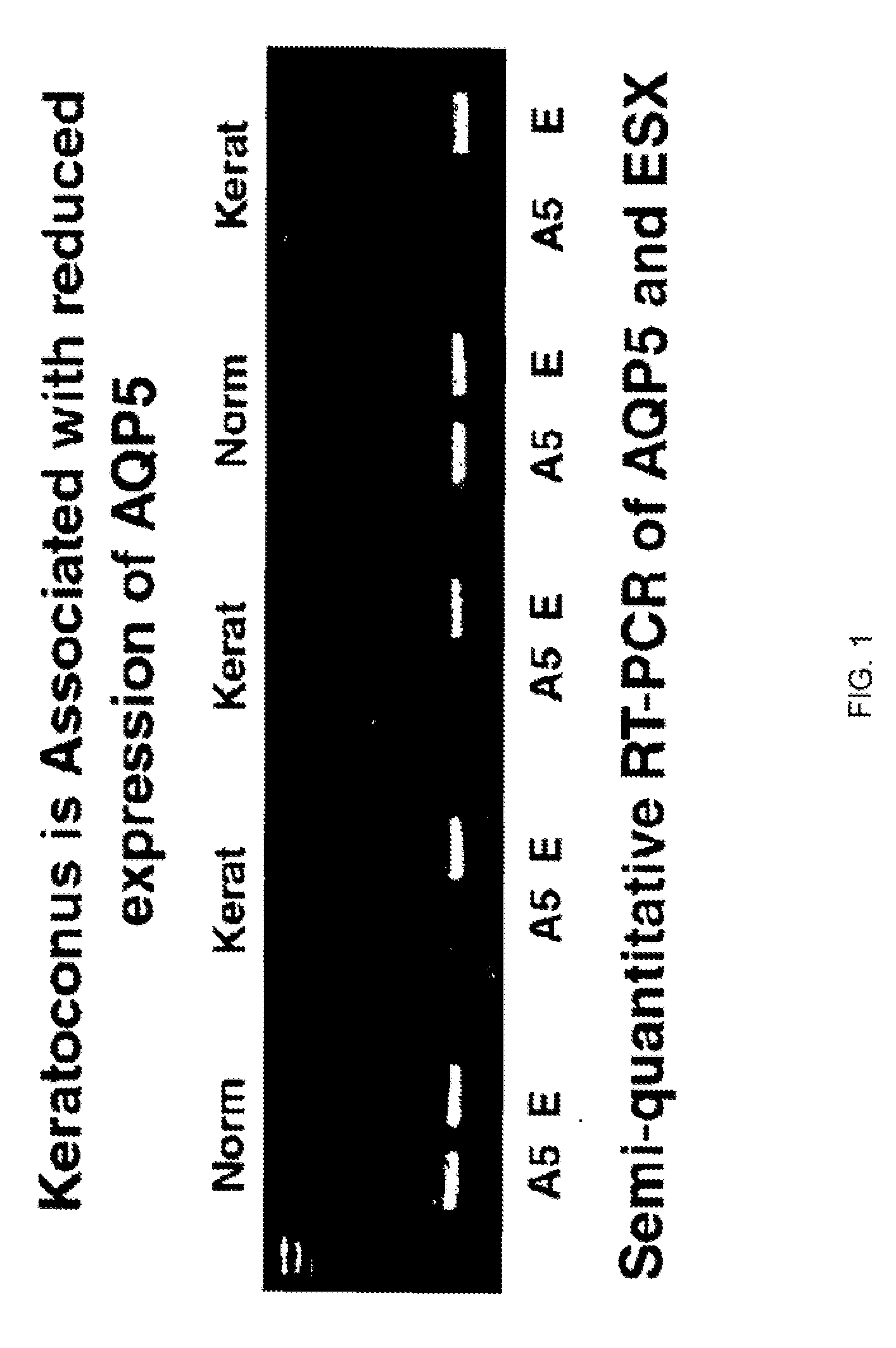
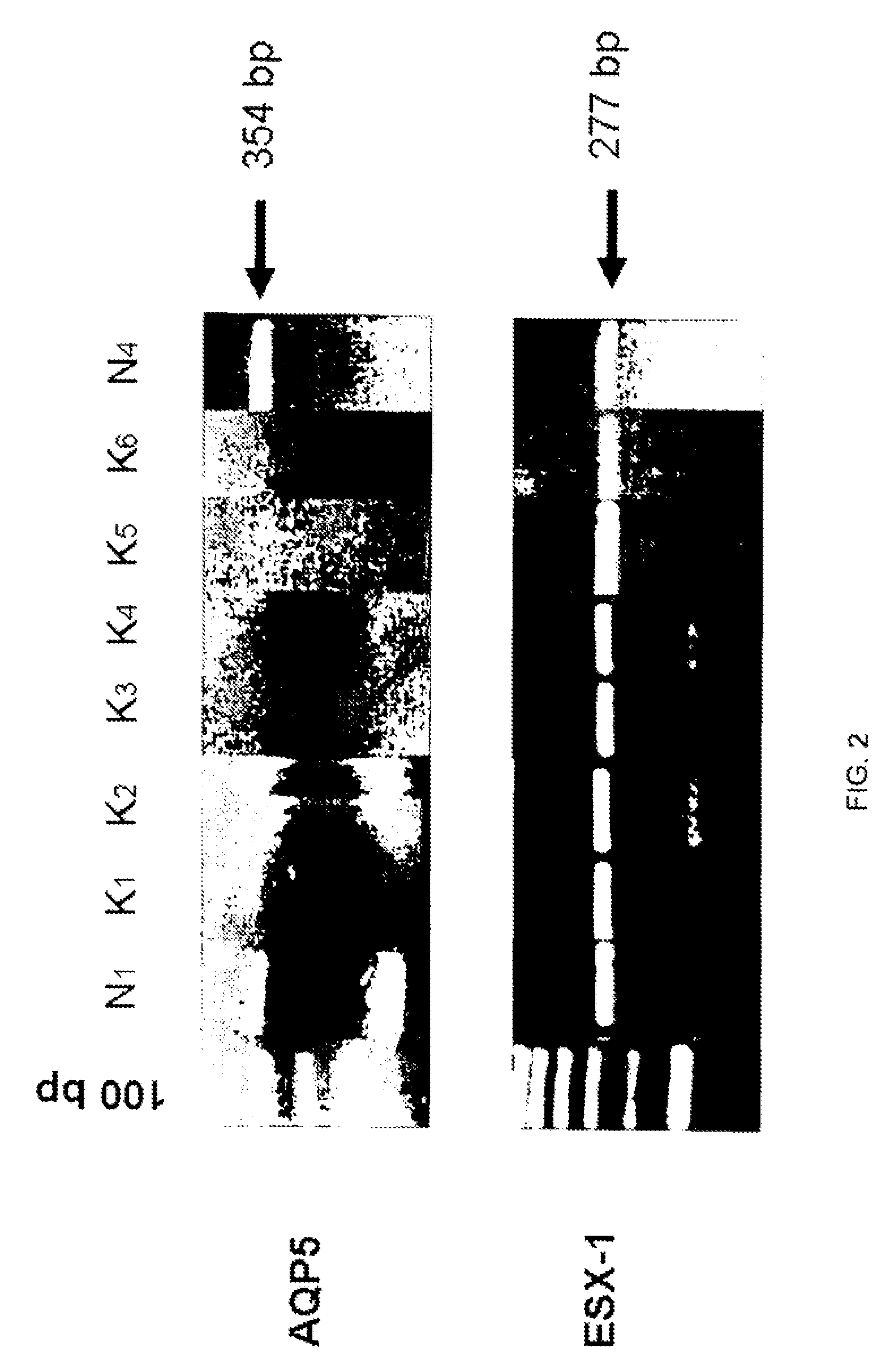
Compositions and methods for detecting keratoconus
InactiveUS20070248970A1Sugar derivativesMicrobiological testing/measurementConical corneaSub clinical
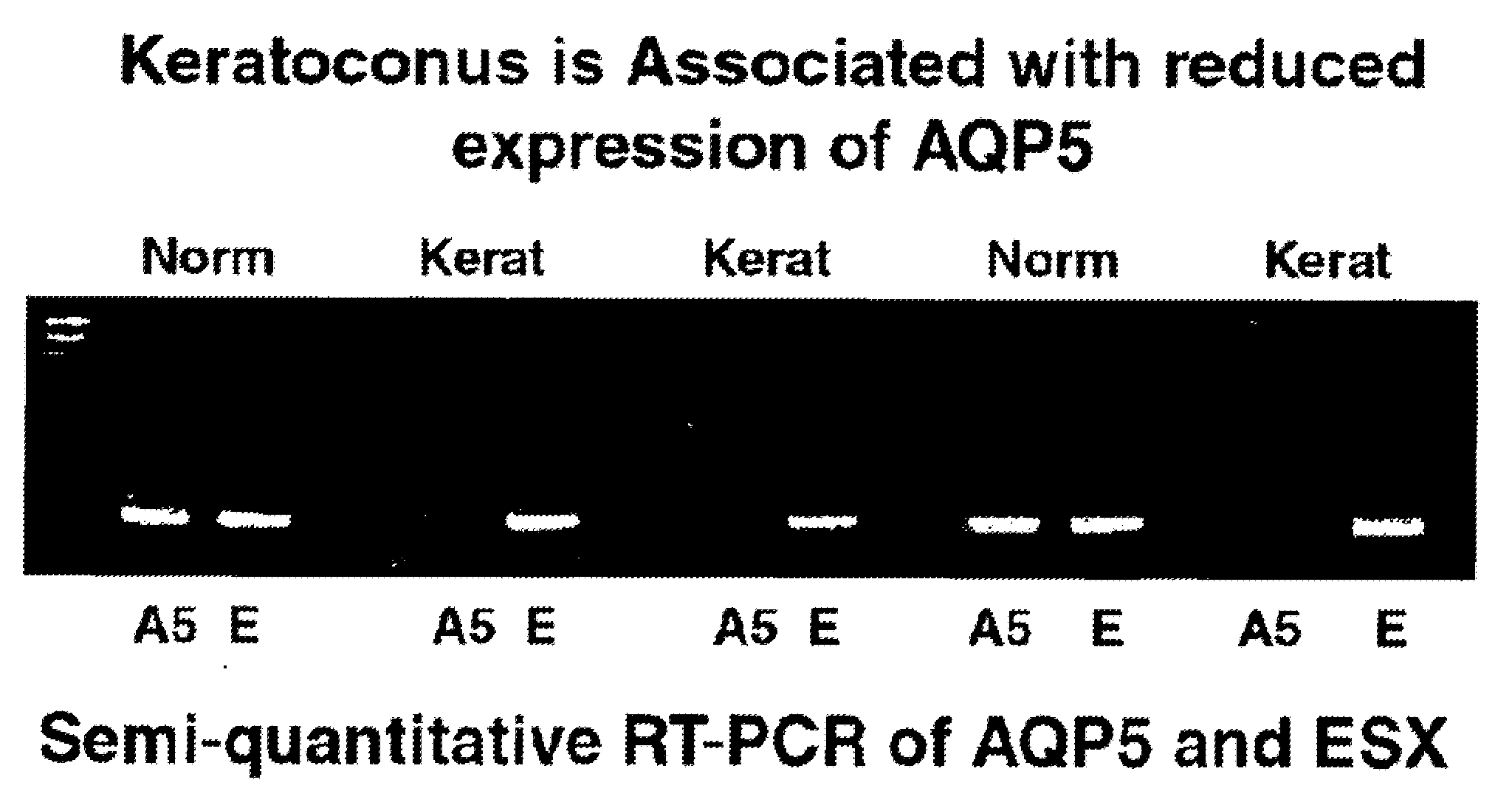
The invention provides a method and assay kits for detecting keratoconus, including sub clinical keratoconus, in a specimen. The method comprises assaying a specimen of corneal epithelium for the presence of an expression product of the AQP5 gene. The invention further provides a novel gene, KC6, that exhibits cornea-specific expression, as well as KC6-related molecules.
Owner:EYE BIRTH DEFECTS RES FOUND
A method for preparing bioactivity possessed artificial cornea
The invention relates to a method for preparing biologically active artificial cornea. It takes animal cornea basic material as raw material, promoting cornea growth or / and preventing partial lesion by enzyme slaking, repeated unfreezing, washing, and irradiating with 60Co. It employs physical and chemical method to remove cell component and soluble protein which will cause immune response, and retains external base material construction, adds multifunctional component which is favor for cornea cell growth or / and preventing partial lesion, freeze dries and stores it. The tensile strength and diopter of prepared artificial cornea are similar to that of normal cornea, it can prevent lesion and dissolution after being implanted in, and promote cornea epithelium regeneration and collagen synthesis; the biocompatibility is good, no obvious immune rejection reaction and no toxic to cell, and can be used to treat various eye injury.
Owner:西安组织工程工程技术研究中心
Low antigen hetero stroma of cornea treated by frozen, and its prepn. method
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
Preservative-free ophthalmic in-situ gelling agent and preparation method thereof
InactiveCN102198087AExtended stayGood bioadhesionOrganic active ingredientsSenses disorderSolubilityCorneal epithelium
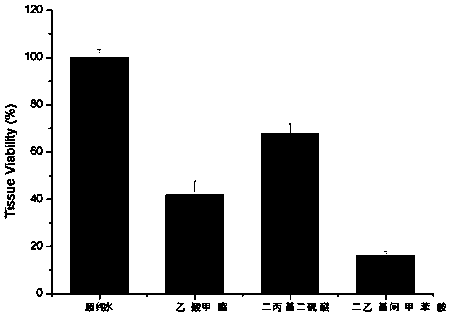
The invention discloses a preservative-free ophthalmic in-situ gelling agent and a preparation method thereof. The preservative-free ophthalmic in-situ gelling agent comprises Carbomer, chitosan, lubricant, stabilizer, thickener, antioxidant, acetic acid, pH regulator, osmoregulator and de-ionized water. By using the Carbomer and chitosan as base materials for the preparation of the in-situ gelling agent, the in-situ gelling agent increases the solubility of the medicament, improves the absorption and bioavailability of the medicament, reduces the frequency of use and enhances the safety and efficiency; by containing the chitosan, the in-situ gelling agent has biological adhesive action, so that the adhesion of medicament particles onto the cornea is enhanced; and the in-situ gelling agent is free of preservative, thereby having no stimulus or damage to the corneal epithelium. The preservative-free ophthalmic in-situ gelling agent can be used as artificial tear for treating xerophthalmia, and can be also used as an administration and transmission system of ophthalmic medicaments.
Owner:SOUTH CHINA UNIV OF TECH
Method of Tissue-Selective Targeted Gene Transfer
The present invention relates to methods of delivering a gene, such as a therapeutic gene, to a desired area of stroma of a cornea that involves removing the corneal epithelium and dehydrating the cornea. Certain aspects of the present invention relate to methods of treating corneal scarring by delivering a TGFβ-antagonizing gene packaged in a viral vector.
Owner:UNIVERSITY OF MISSOURI
Ophthalmic medicament composition for forming low-irritation transparent emulsion formulation for surface immune adjustment and inflammation reduction of relevant tissue of eyes or eye periphery
ActiveCN101897949AImprove solubilityLess irritatingSenses disorderCyclic peptide ingredientsPolyoxyethylene castor oilKERATOCONJUNCTIVITIS SICCA
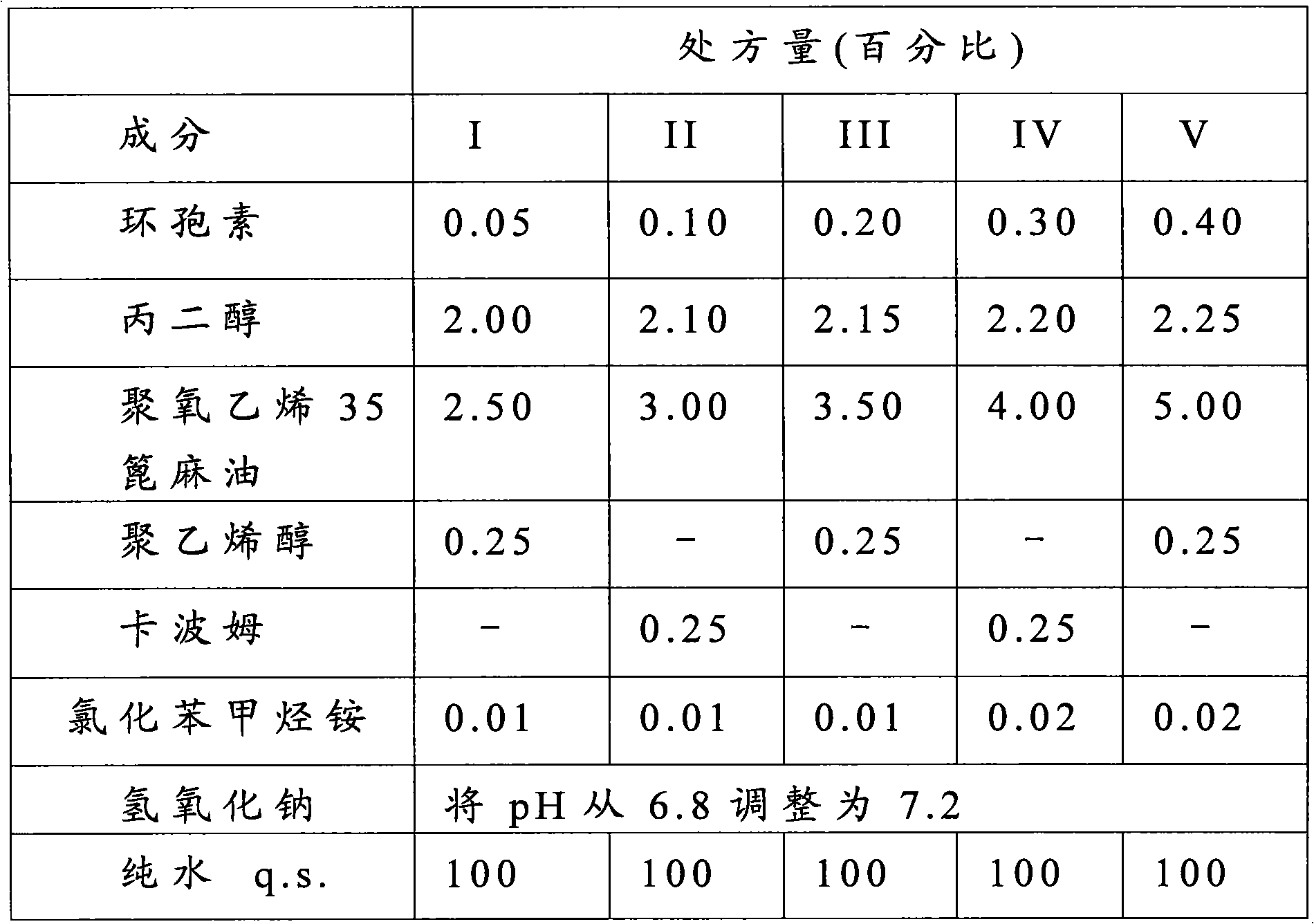
The invention provides an ophthalmic medicament composition for forming a low-irritation transparent emulsion formulation for the surface immune adjustment and inflammation reduction of the relevant tissue of eyes or eye periphery. The ophthalmic medicament composition is used for treating the inflammatory reaction of a serious keratoconjunctivitis sicca and cornea epithelium pathological change patient, forms an emulsion formulation, contains at least one ciclosporin, propylene glycol and polyoxyethylene castor oil derivative, such as polyoxyethylene 35 castor oil derivative, and the like, is transparent, has the characteristics of low irritation, stability and no crystallization phenomenon and is suitable for more sensitive areas, such as eye tissue, and the like.
Owner:RXVISION PHARMA CO LTD
Cornea-like epithelioid cell, tissue-engineered corneal epithelium as well as preparation and application
ActiveCN109517784AGood transparencyGood elasticityEpidermal cells/skin cellsNervous system cellsLimbal stem cell deficiencyLimbal stem cell
The invention belongs to the technical field of tissue engineering and in particular relates to a method and application for constructing tissue-engineered corneal epithelium by taking cornea-like epithelioid cells as seed cells. The invention provides a two-step method for inducing skin-derived epidermic cells to be transformed and differentiated into the cornea-like epithelioid cells, so that the cornea-like epithelioid cells obtained by induction serving as the seed cells replace the corneal limbal stem cells for constructing the tissue-engineered corneal epithelium. The prepared tissue-engineered corneal epithelium has the characteristics of being excellent in transparency, excellent in elasticity, high in operability and the like, can be applied to transplantation and repair of ocularsurfaces of animals or animals with severe vision losses or blindness due to corneal limbal stem cell deficiency, and can achieve excellent effects when applied to autotransplantation and allotransplantation of blind animal models due to the corneal limbal stem cell deficiency.
Owner:LUOYANG NORMAL UNIV
Method for treating pterygium
Owner:王晋平
Mixture ophthalmic strips
InactiveUS20150168380A1Microbiological testing/measurementDisease diagnosisXerophthalmiaBulbar conjunctiva
This invention is within the field of eye medicine, involving the preparation method of one type of cornea intravital staining. The above mentioned staining includes the mixed solution of fluorescein sodium aqueous solution and lissamine green aqueous solution. In the above fluorescein sodium aqueous solution, the concentration of fluorescein sodium is 0.5%-4.0% w / v; while in the above lissamine green aqueous solution, the concentration of lissamine green is 0.5%-4.0% w / v. And the volume ratio of the above mentioned fluorescein sodium aqueous solution and lissamine green aqueous solution is 1:0.25-1:3. The cornea intravital staining in this invention possesses some advantages like making the staining of cornea and bulbar conjunctiva proceed at the same time, completed by one time, little irritation to eye tissue and being susceptive for patients. And it is mainly used in diagnosing and evaluating xerophthalmia, keratohelcosis, keratitis (KCS), arborized corneal epithelium herpes, and early diagnosis of Sjogren syndrome etc.
Owner:WU LIANG
Compound dendrobium eye drops for treating xerophthalmia and preparation method thereof
InactiveCN105726983ARich sources of medicineLow costSenses disorderPharmaceutical delivery mechanismTreatment effectCornea epithelium
The invention belongs to the traditional Chinese medicine field, relates to a traditional Chinese medicine prescription for treating xerophthalmia and a preparation method, and more specifically to a compound dendrobium eye drops for treating xerophthalmia and a preparation method thereof. The compound dendrobium eye drops are prepared from the following raw materials: 80-120g of caulis dendrobii, 80-120g of radix trichosanthis, 80-120g of folium isatidis, 50-100g of vitamin B1, and 10-20ml of honey. The eye drops are prepared by a modern advanced medicine pharmaceutical technology and have a reasonable formula which is scientific and advanced. The medicinal herb resource is abundant and the medicine cost is low. The traditional Chinese medicine prescription for treating xerophthalmia has the efficacy of improving eyesight and anti-inflammation, lubricating eye surface, and prompting cornea epithelium growth. A local drug delivery mode is employed, and the medicines directly reach the action position. The compound dendrobium eye drops for treating xerophthalmia provides a safe and effective eye medicinal preparation with convenient use for clinic treatment of the xerophthalmia. The treatment effect is reliable, and the compound dendrobium eye drops for treating xerophthalmia and the preparation method thereof can be popularized.
Owner:QINGDAO HUANGDAO HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1218755CGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
Owner:北京科宇联合干细胞生物技术有限公司
Eye washing liquid for preventing xerophthalmia and preparation method thereof
InactiveCN110960600ARich sources of medicineLow costSenses disorderHydroxy compound active ingredientsMedicinal herbsFormulary
The invention discloses eye washing liquid for preventing xerophthalmia and a preparation method thereof. The eye washing liquid is prepared from the following raw materials: 60g to 80g of cornflowers, 80g to 100g of folium isatidis, 20g to 40g of herba menthae, 20g to 30g of vitamin A, 6ml to 8ml of honey and 6ml to 8ml of pearl liquid. The eye washing liquid is prepared by adopting the modern advanced medical pharmaceutical technology, is reasonable in formula, and has scientificity and advancement; the selected materials are rich in medicine source; the medicinal materials are low in cost;the eye washing liquid has effects of improving eyesight, resisting to inflammation, lubricating the ocular surface, prompting corneal epithelium to grow; a local administration mode is adopted; medicine directly reaches an acting position; an eye medicinal preparation which is safe, effective and convenient to use is provided for clinical treatment on xerophthalmia; and the eye washing liquid isreliable in treatment effect and has generalizability.
Owner:重庆视健眼健康产业有限公司
A method for preparing bioactivity possessed artificial cornea
The invention relates to a method for preparing biologically active artificial cornea. It takes animal cornea basic material as raw material, promoting cornea growth or / and preventing partial lesion by enzyme slaking, repeated unfreezing, washing, and irradiating with 60Co. It employs physical and chemical method to remove cell component and soluble protein which will cause immune response, and retains external base material construction, adds multifunctional component which is favor for cornea cell growth or / and preventing partial lesion, freeze dries and stores it. The tensile strength and diopter of prepared artificial cornea are similar to that of normal cornea, it can prevent lesion and dissolution after being implanted in, and promote cornea epithelium regeneration and collagen synthesis; the biocompatibility is good, no obvious immune rejection reaction and no toxic to cell, and can be used to treat various eye injury.
Owner:西安组织工程工程技术研究中心
Low antigen hetero stroma of cornea treated by frozen, and its preparation method
InactiveCN100386061CRetain toughnessGood biocompatibilityEye implantsTransplanted corneaCorneal endothelium
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
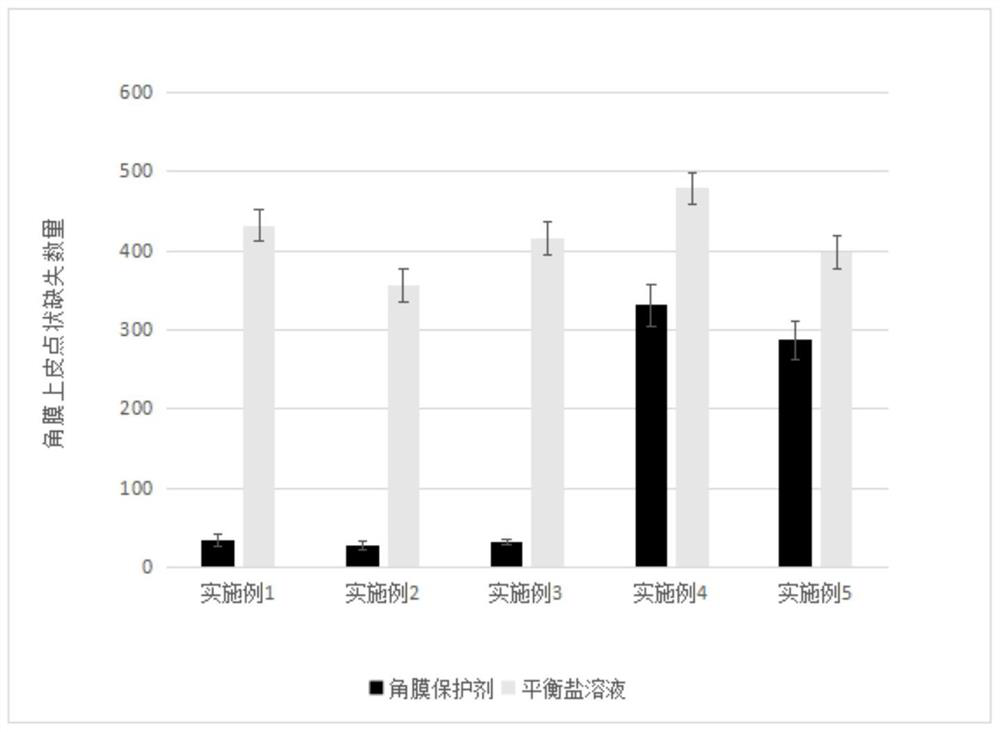
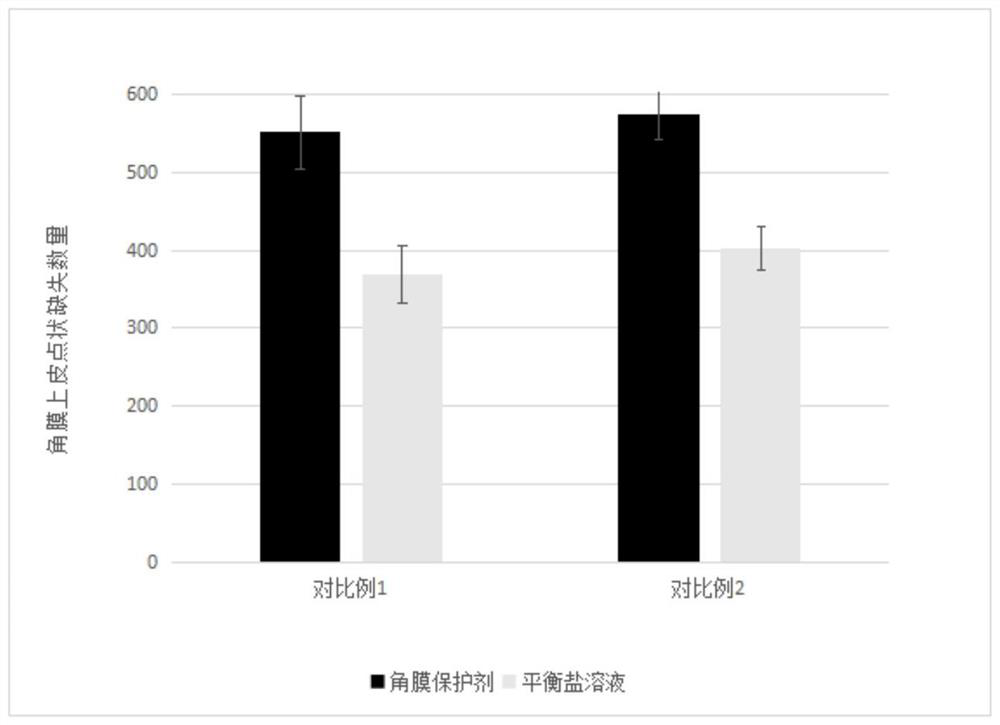
Cornea protective agent as well as preparation method and application thereof
PendingCN113876800AHigh transparencyReduced risk of epithelial dysfunctionOrganic active ingredientsSenses disorderSurgeryMethyl palmoxirate
The invention provides a cornea protective agent. The cornea protective agent comprises hydroxypropyl methyl cellulose with the viscosity of 5mPa.S to 200,000 mPa.S and / or chondroitin sulfate with the molecular weight of 20,000 to 500,000, an anti-inflammatory substance and a buffer solution. The cornea protective agent disclosed by the invention can protect the cornea surface in an operation, can keep the cornea surface moist within a certain time and keep good transparency of the cornea, and provides a good operation visual field. Meanwhile, the risk of postoperative corneal epithelium dysfunction can be reduced, and postoperative complications are reduced.
Owner:TIANJIN JINGMING NEW TECH DEV CO LTD
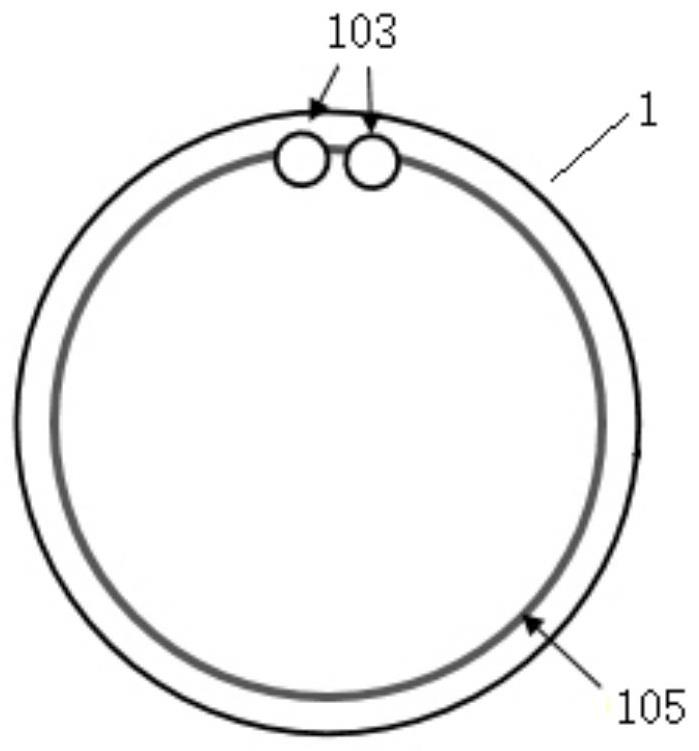
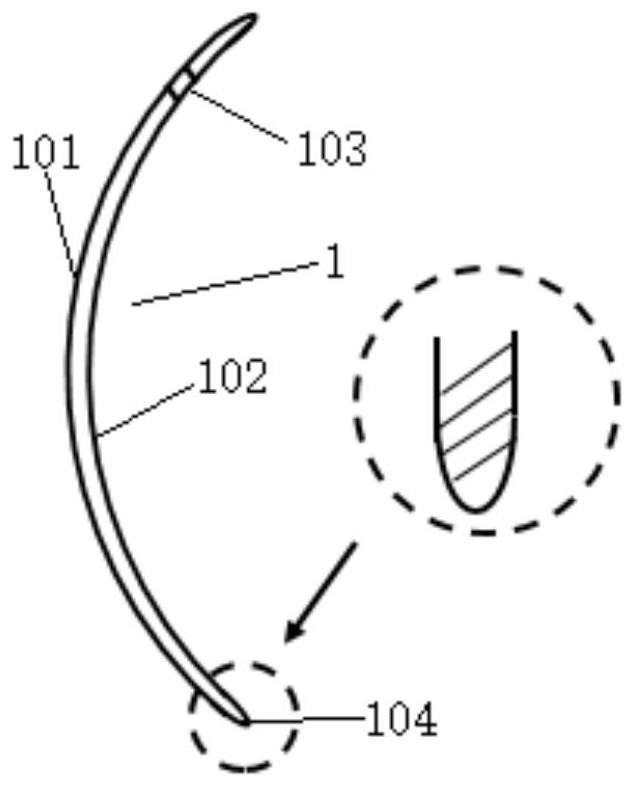
Cornea protection glasses for animals
PendingCN114176895AReduce stimulationPrevent secondary scratchesEye treatmentCorneal surfaceCorneal drying
The invention discloses animal cornea protection glasses which comprise lenses, the lenses are in a concave-convex lens shape and are provided with smooth inner surfaces and outer surfaces, the outer surfaces of the lenses are convex surfaces, the inner surfaces of the lenses are concave surfaces, the lenses are composed of inner lens layers and outer lens layers, and colorful ring bands are filled between the inner lens layers and the outer lens layers at the edges of the lenses in a film pressing mode. Two round, square or irregular small holes are further formed in the edge of the lens, the lens is made of a soft hydrophilic material with high oxygen permeability and high water content, and the oxygen permeability coefficient DK is smaller than ISO; the soft contact band-aid and the therapeutic lens are specially designed for animals, are soft contact band-aid and the therapeutic lens, can prevent the cornea from being dry or stimulated and prevent pets from touching the cornea when being worn on the surface of the animal cornea, and can be used for preventing the cornea from being damaged. Meanwhile, therapeutic drugs can be dropwise added between the lens and the cornea, so that corneal epithelium regeneration is promoted, and pain is relieved.
Owner:TIANJIN SHI JI KANG TAI BIOMEDICAL ENG CO LTD
Corneal neovascularization/lymphatic vessel generation injury model and construction method and application thereof
PendingCN113520656AEasy to buildEfficient build methodCompounds screening/testingSurgical veterinaryLymphatic vesselOphthalmology
The invention provides a corneal neovascularization / lymphatic vessel generation injury model and a construction method and application thereof. The model is constructed on the basis of a corneal suture thought by combining mechanical scraping of corneal epithelium and bonding of the corneal suture to the corneal stroma by biological glue, so that the problems of high modeling difficulty and low efficiency when the corneal suture model is applied to small rodents are solved; and common reasons such as cornea perforation and suture shedding which cause modeling failure are avoided, and the advantages of regular growth of cornea suture neovascularization, avoidance of chemical reagent influence in a chemical induction method, single and controllable injury mode and the like are reserved. Besides, based on the construction method of the model, the model can be simply, effectively and controllably used as a novel effective model for mouse and rat corneal neovascularization or lymphatic vessel generation, so that the model is relatively high in applicability.
Owner:GENERAL HOSPITAL OF PLA
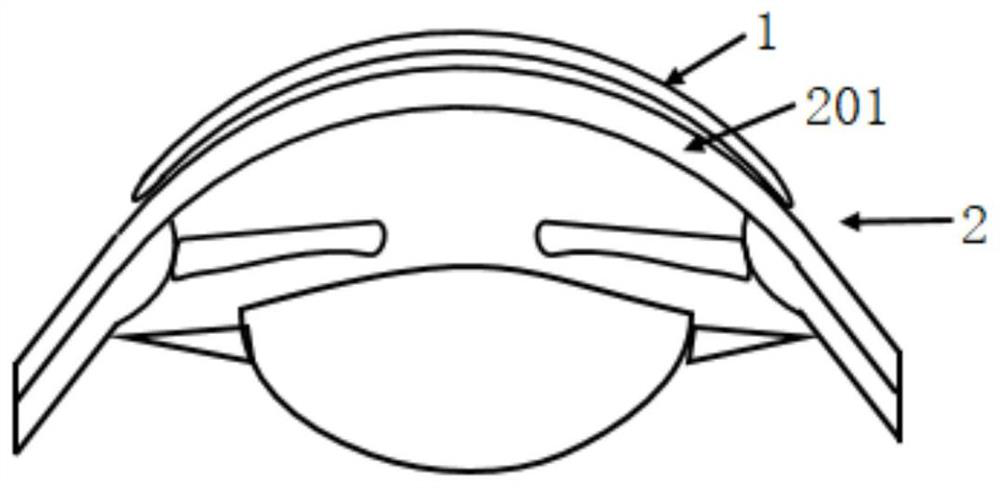
Corneal bandage lens and preparation method thereof
PendingCN111481340AClear protective effectProlonged clarityLaser surgeryProsthesisVisual field lossVitreous surgery
A corneal bandage lens prepared by the preparation method of the corneal bandage mirror provided by the embodiment of the invention has the advantages that the structure is simple; when the corneal bandage lens is applied to vitreous surgeries, in the surgery, the visual field of doctors is kept clear for a long time; generally greater than 10 minutes, if sharpness has decreased, a small amount ofbalanced salt solution is utilized; washing the surface of the corneal bandage lens; and when the sclera is pressed and the eyeball is rotated, the visual field of the doctor is not obviously deformed in the operation, and after the existing corneal bandage mirror is improved, the corneal bandage mirror is applied to the vitreous operation, so that the burden of an assistant is greatly reduced, the operation efficiency is improved, and the clinical application range of the existing corneal bandage mirror for treatment is expanded. Meanwhile, it is found that after the corneal bandage lens isremoved after the surgery is finished, the corneal epithelium integrity and the corneal epithelium edema degree of the operation eye are superior to those of a traditional balanced salt solution flushing set, and therefore the existing corneal bandage lens for treatment also has a clear protection effect on corneas in vitreous body operation.
Owner:杨晓岗
A type of corneal epithelial cells, tissue engineering corneal epithelium, preparation and application
ActiveCN109517784BHigh transparencyIncrease elasticityEpidermal cells/skin cellsNervous system cellsAllotransplantationCorneal epithelial cell
Owner:LUOYANG NORMAL UNIV
Method for inducing human epidermal stem cells into corneal epithelial cells
ActiveCN109517051BHigh activityHigh differentiation efficiencyEpidermal cells/skin cellsNervous system cellsCorneal epithelial cellCornea epithelium
The invention provides a method for inducing human epidermal stem cells into corneal epithelioid cell differentiation in vitro. A specific cornea promoting peptide is used, so that the differentiationefficiency of the cornea epithelial cells can be obviously improved.
Owner:RAFAEL (SHENZHEN) INVESTMENT CONSULTING CO LTD
Marker pen for implanting astigmatism correction type intraocular lens
PendingCN112587283ASimple procedureShorten operation timeIntraocular lensAstigmatism correctionSyringe needle
The invention discloses a marker pen for implanting an astigmatism correction type intraocular lens. The marker pen comprises a pen body, a coloring part and a cutting part, wherein dye is contained in the pen body; the coloring part is arranged at the end part of the pen body, so that the dye flows out from the coloring part; and the cutting part is arranged at the front end of the coloring part.A medical worker can complete cutting operation of corneal epithelium and the clear and fine marking operation of marking points A and B by independently using the marking pen, and does not need to cut the corneal epithelium by using a syringe needle and then perform coloring marking by using the marking pen, so that the operation steps of implanting the astigmatism correction type intraocular lens are simplified, and the operation time is shortened.
Owner:XIAMEN EYE CENTER OF XIAMEN UNIVERSITY CO LTD
Recombinant cornea model for ocular surface irritation evaluation and preparation method thereof
PendingCN110066847ASolve non-corneal problemsSolve the problem of difficult expansionEye implantsMicrobiological testing/measurementIrritationOcular surface
The invention relates to a recombinant cornea model for ocular surface irritation evaluation and a preparation method thereof, in particular to the field of in-vitro organ culture and tissue regeneration. From the perspective of seed cells, by constructing the three-dimensional corneal epithelium model through in-vitro enrichment and corneal limbal stem cell amplification, the in-vitro in-vivo consistency of eye irritatwion evaluation is ensured with the seed cells as a cornea family, the problem about industrial large-scale production is also solved, and the promotion of ocular surface irritation in-vitro substitutive experiments is facilitated.
Owner:GUANGDONG BOXI BIO TECH CO LTD
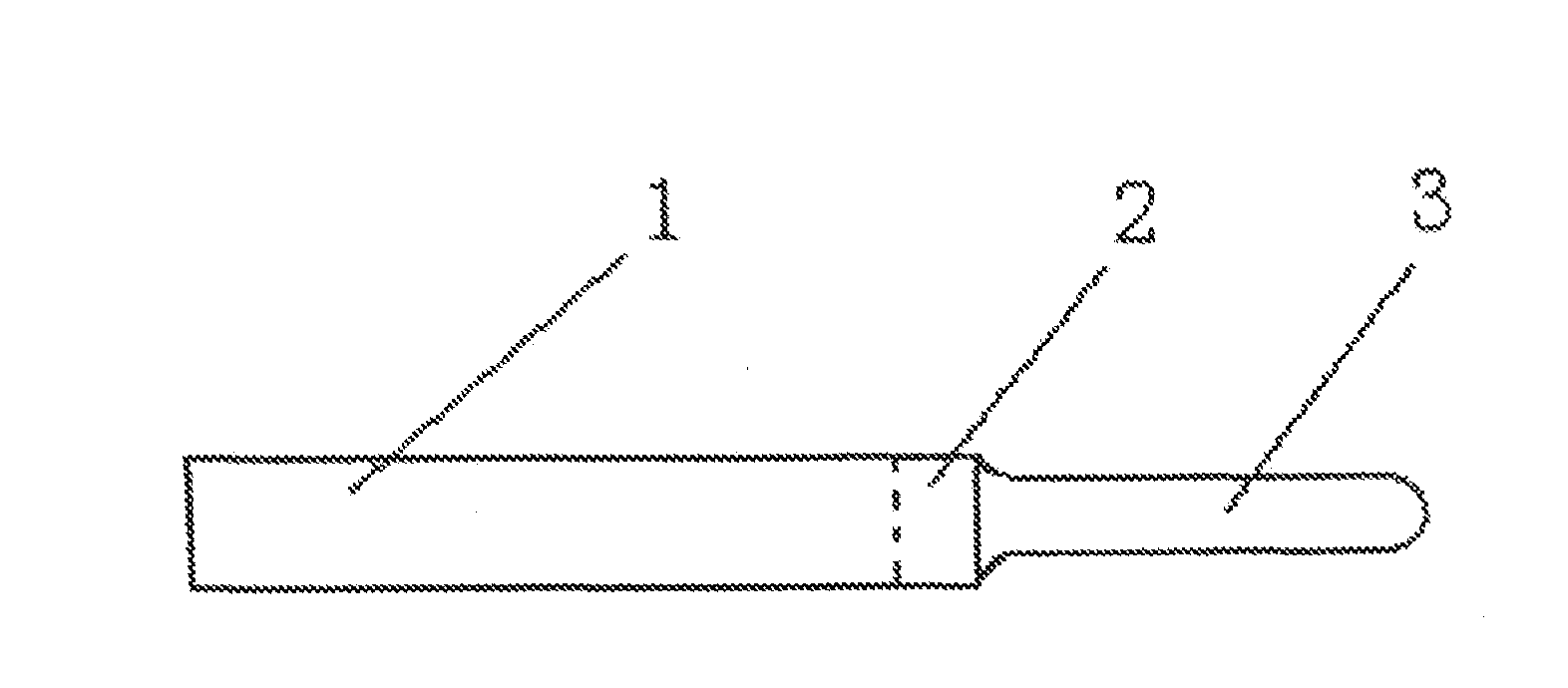
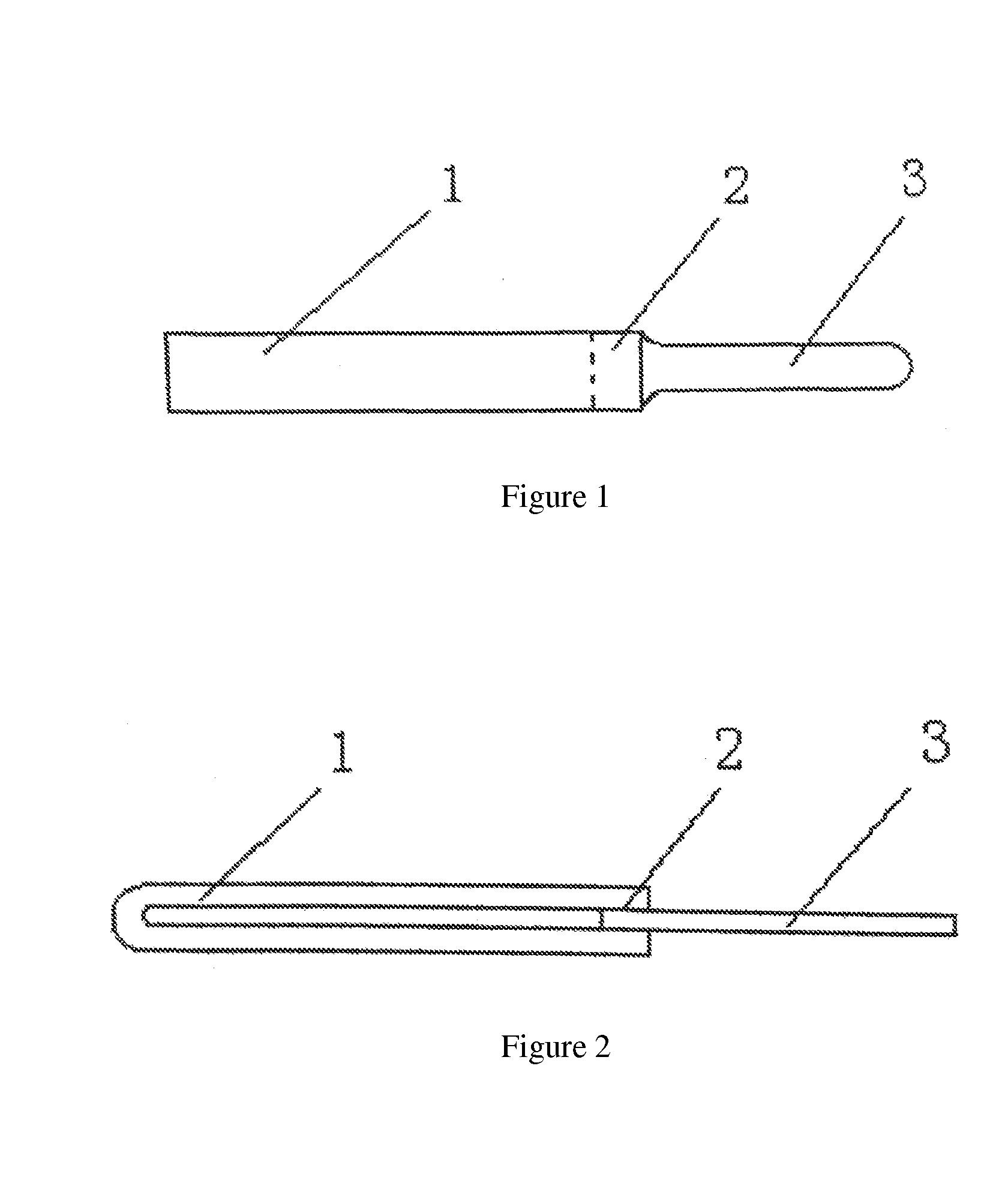
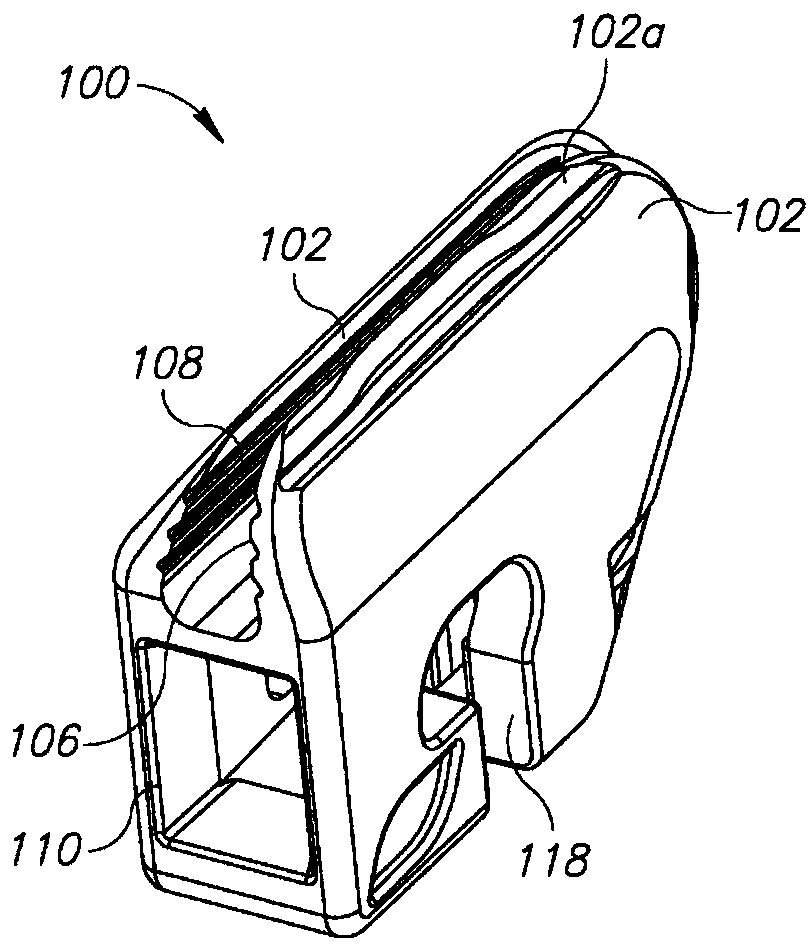
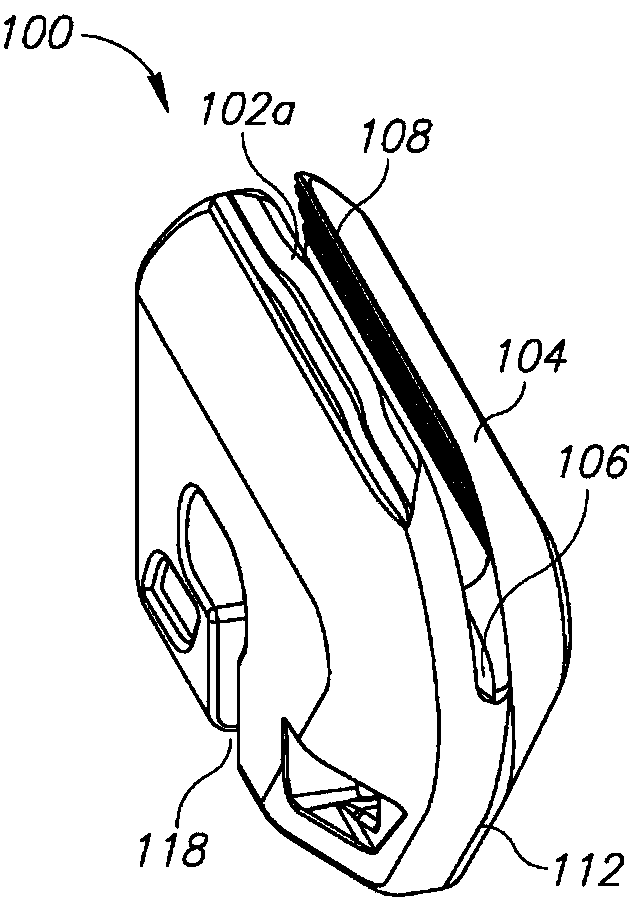
Devices and methods for removing corneal epithelium
A device is disclosed comprising: an elongated head having a distal end formed by an elongated edge of a cutting blade disposed parallel to an elongated edge of a control blade, wherein the elongated edges of the corresponding control and cutting blades Separated by a uniform gap, forming a distal opening in communication with a passage extending from the front end to the rear end of the head, wherein the elongated edge of the cutting blade extends distally beyond the elongated edge of the control blade such that the edge of the cutting blade meets the control blade. providing a height difference between the edges of the blades, wherein the edges of the cutting blade are sharp and configured to cut body tissue, and wherein the edges of the control blade are blunt and form a barrier to limit the edge penetration of the cutting blade to the depth of the body tissue.
Owner:ORCA SURGICAL
Opthalmic compositions comprising viscosifying polymers and nucleic acids
PendingUS20220265695A1Prevents and reduces RNA toxicityPrevents and diminishes formationOrganic active ingredientsOintment deliveryDescemets MembraneCorneal disease
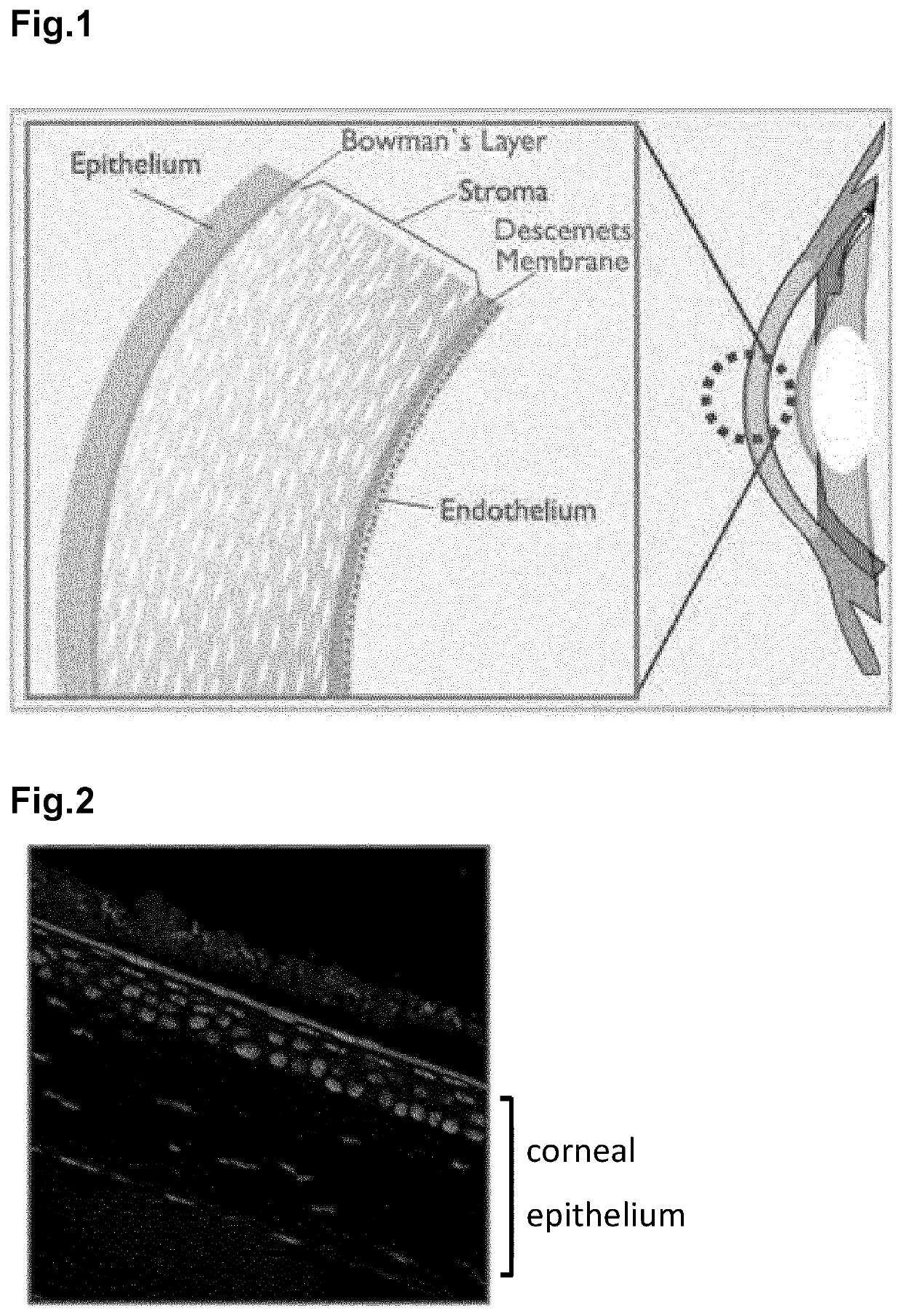
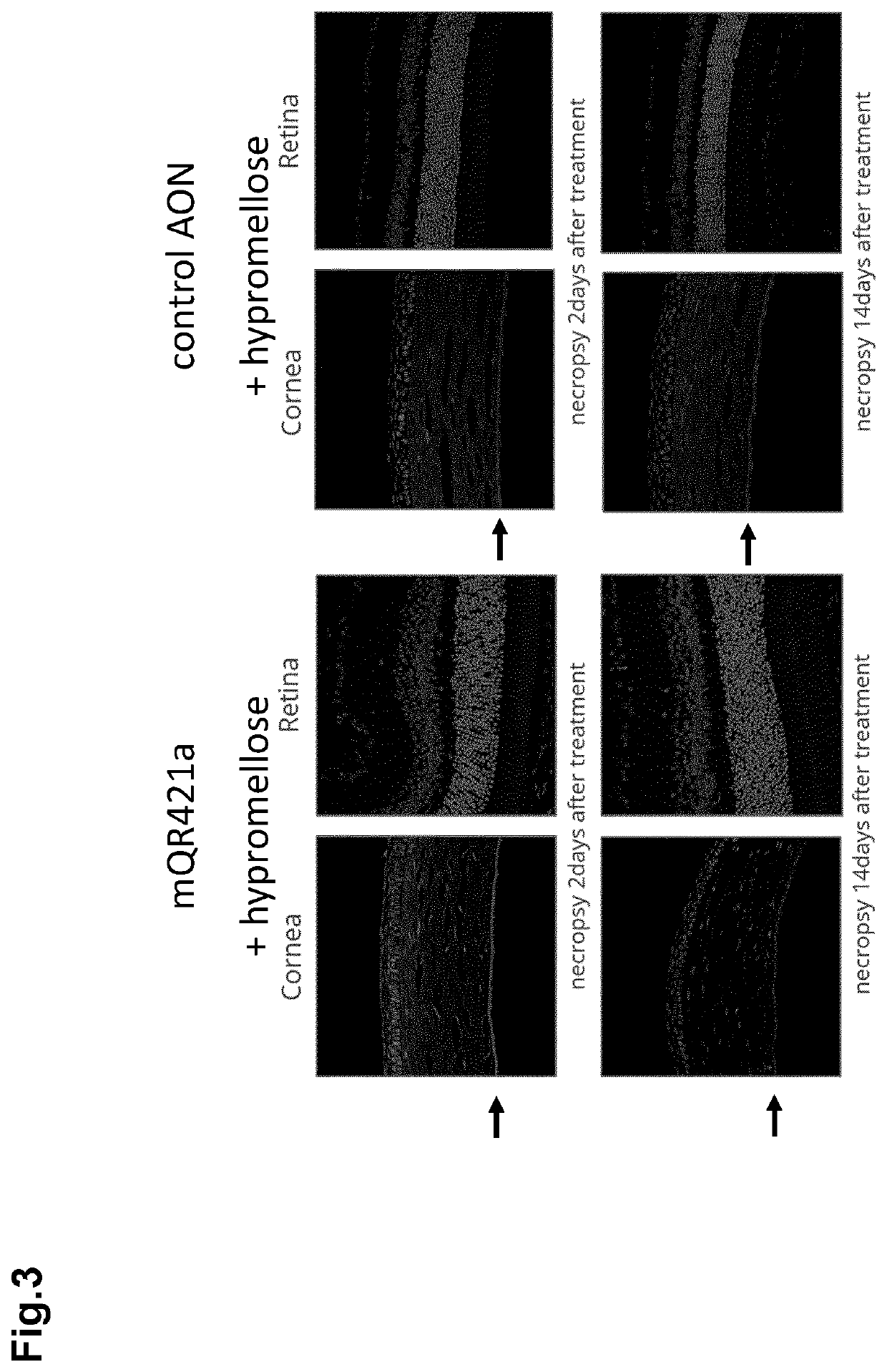
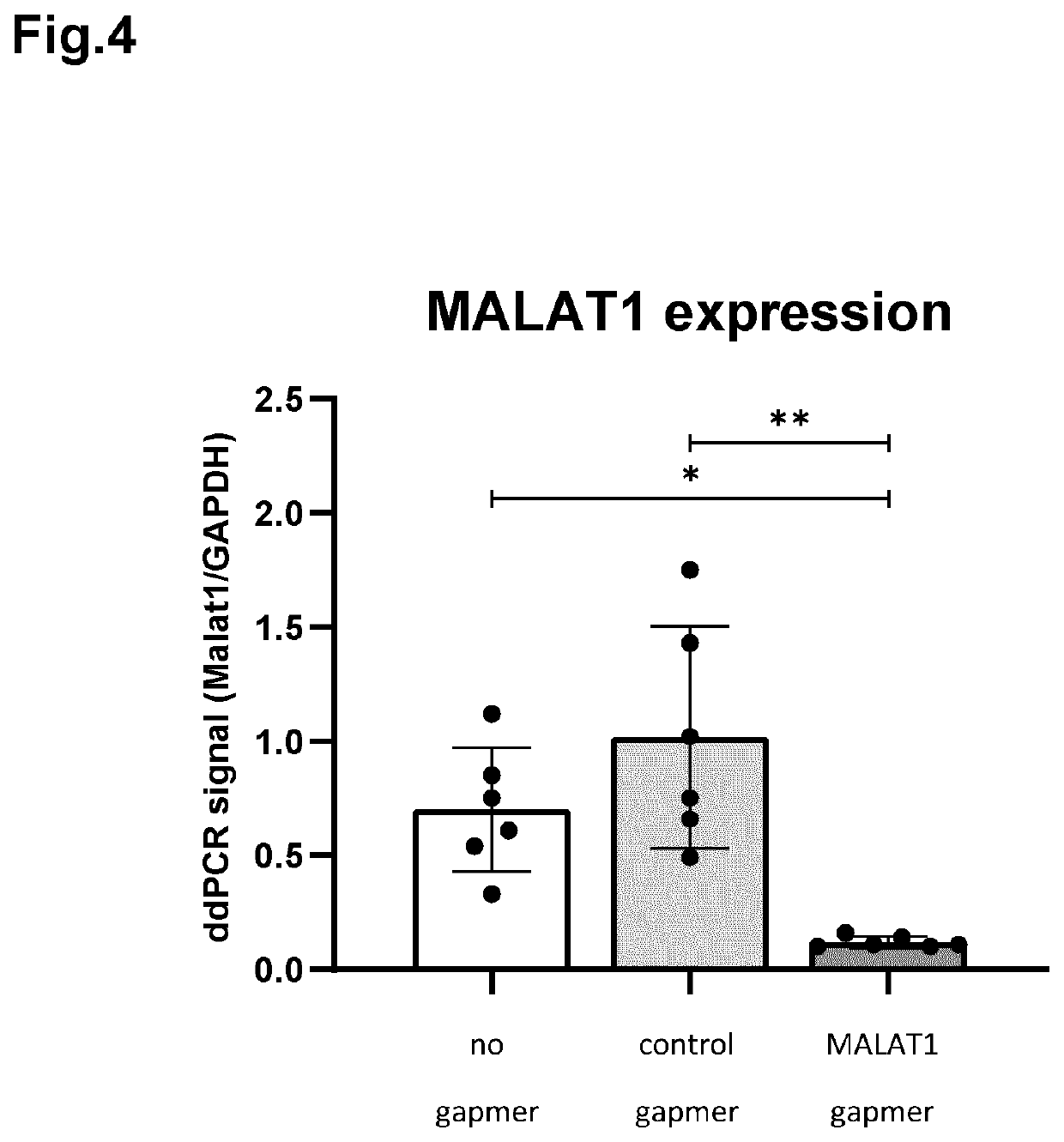
The invention relates to ophthalmic compositions comprising: i) a nucleic acid molecule, preferably an antisense oligonucleotide, such as an single-stranded antisense oligonucleotide that modulates splice modulation or prevention of RNA toxicity due to trinucleotide repeats in a target RNA molecule, or a gapmer that induces breakdown of a target RNA molecule after formation of a double-stranded RNA / gapmer complex; and ii) a viscosifying polymer. The ophthalmic compositions are for topical administration in the eye of a mammalian subject suffering from a corneal disease, such as a hereditary corneal dystrophy. The viscosifying polymer in the compositions of the invention allows the entry of the nucleic acid molecule to the different layers of the cornea: the corneal epithelium, Bowman's membrane, stroma, Dua's layer, the Descemet's membrane and / or the corneal endothelium.
Owner:PROQR THERAPEUTICS II BV
Preservative-free ophthalmic in-situ gelling agent and preparation method thereof
InactiveCN102198087BPromote absorptionImprove bioavailabilityOrganic active ingredientsSenses disorderPharmaceutical SubstancesOPHTHALMOLOGICALS
The invention discloses a preservative-free ophthalmic in-situ gelling agent and a preparation method thereof. The preservative-free ophthalmic in-situ gelling agent comprises Carbomer, chitosan, lubricant, stabilizer, thickener, antioxidant, acetic acid, pH regulator, osmoregulator and de-ionized water. By using the Carbomer and chitosan as base materials for the preparation of the in-situ gelling agent, the in-situ gelling agent increases the solubility of the medicament, improves the absorption and bioavailability of the medicament, reduces the frequency of use and enhances the safety and efficiency; by containing the chitosan, the in-situ gelling agent has biological adhesive action, so that the adhesion of medicament particles onto the cornea is enhanced; and the in-situ gelling agent is free of preservative, thereby having no stimulus or damage to the corneal epithelium. The preservative-free ophthalmic in-situ gelling agent can be used as artificial tear for treating xerophthalmia, and can be also used as an administration and transmission system of ophthalmic medicaments.
Owner:SOUTH CHINA UNIV OF TECH
Method of treatment using corneal epithelium forming cell sheets
A diseased site where an anterior segment tissue is partly or entirely damaged or deficient can be treated using a corneal epithelium forming cell sheet that will adhere well to the anterior segment tissue. To attain this objective, a corneal epithelium forming cell sheet is produced by a process comprising the steps of cultivating under specified conditions corneal epithelium forming cells on a cell culture support comprising a substrate having its surface covered with a temperature responsive polymer of which the hydrating force varies in a temperature range of 0° C.-80° C., optionally stratifying the layer of cultured cells, and thereafter, (1) adjusting the temperature of the culture solution to either above an upper critical dissolution temperature or below a lower critical dissolution temperature, (2) bringing the cultured corneal epithelium forming cells into close contact with a carrier, and (3) detaching the sheet together with the carrier under specified conditions.
Owner:CELLSEED +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com