Ophthalmic medicament composition for forming low-irritation transparent emulsion formulation for surface immune adjustment and inflammation reduction of relevant tissue of eyes or eye periphery
A composition and drug technology, applied in the field of ophthalmic pharmaceutical compositions, can solve problems such as obstructing vision and unfavorable eyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Embodiments of the present invention are described in detail below, and the embodiments described below are exemplary, and are only used to explain the present invention, but not to limit the present invention.
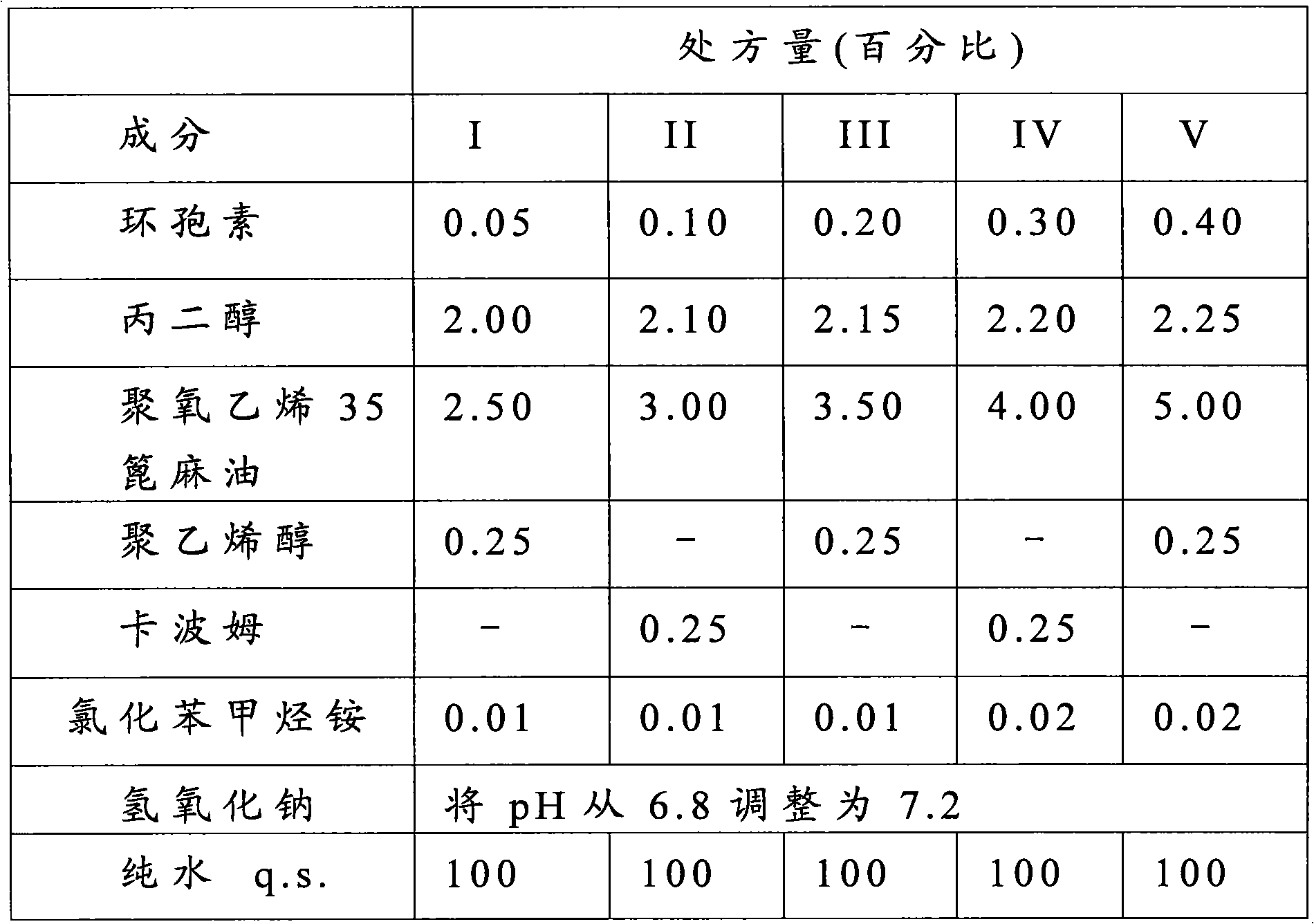
[0018] The present invention is a new ophthalmic pharmaceutical composition, which involves compounds that are not easily soluble in water, such as cyclosporine. The ophthalmic pharmaceutical composition of the present invention is formed as a cyclosporine emulsion containing propylene glycol (propylene glycol) and polyoxyethylene castor oil derivatives, which can provide a high degree of comfort and low irritation.
[0019] The ophthalmic pharmaceutical composition of the present invention is in the form of an ophthalmic emulsion, which is formed as a low-irritant transparent emulsion that is easy to enter into the sensitive visual area, and its components include cyclosporine, propylene glycol, polyoxyalkylene 35 castor oil, polyethylene Alcohol and pure wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com