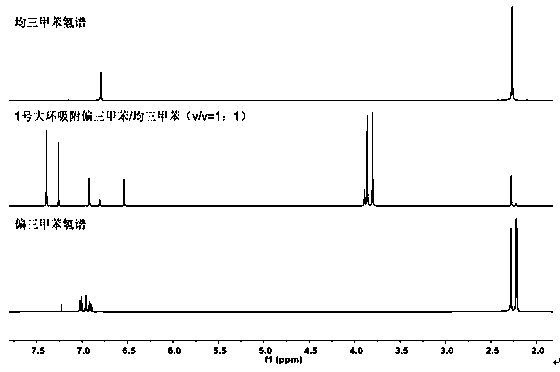
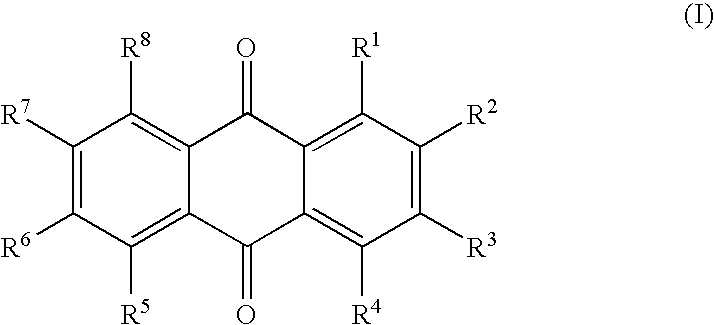
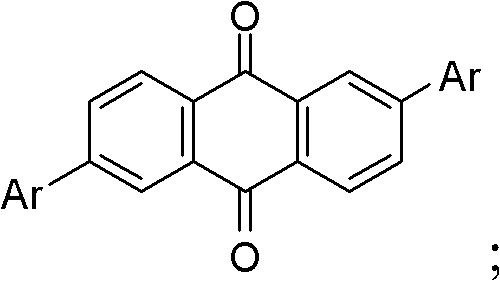
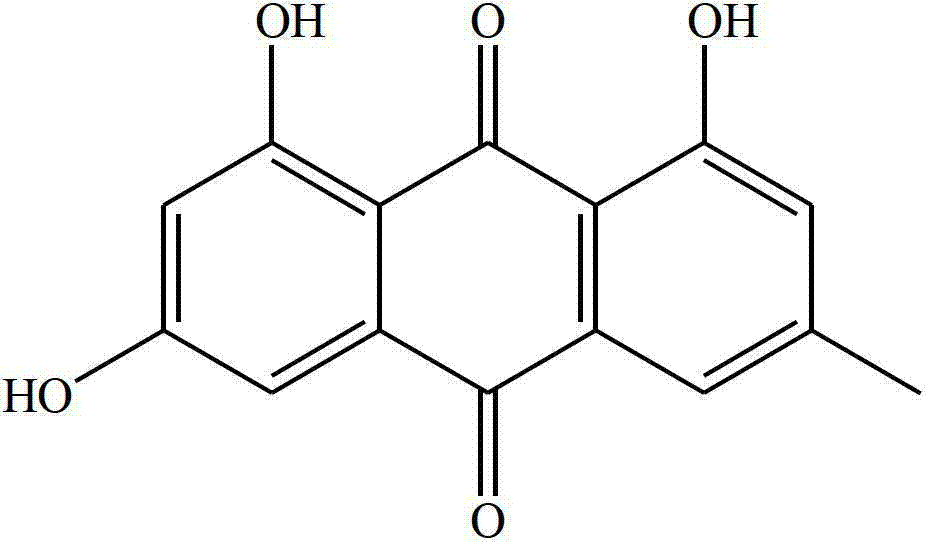
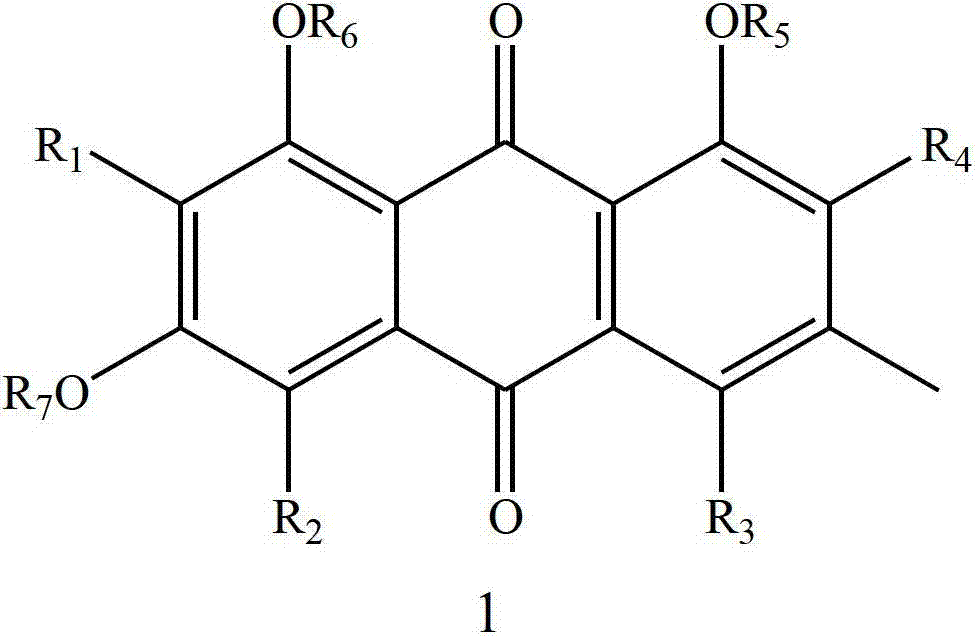
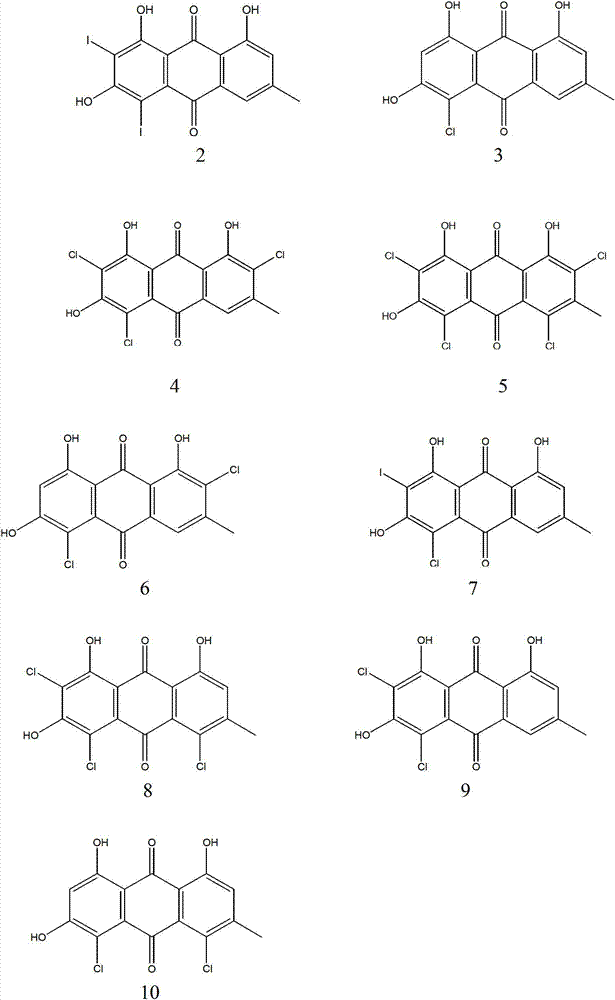
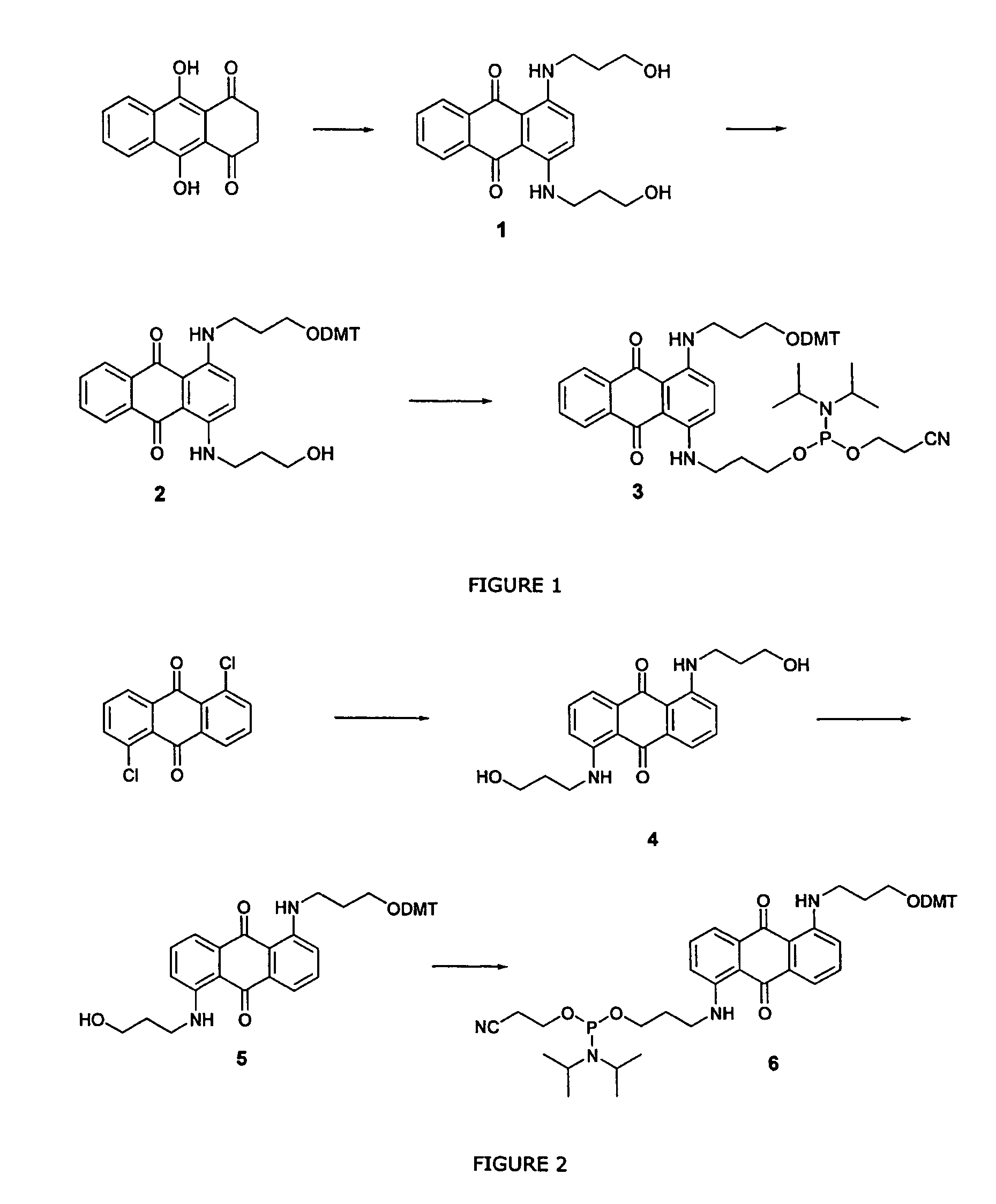
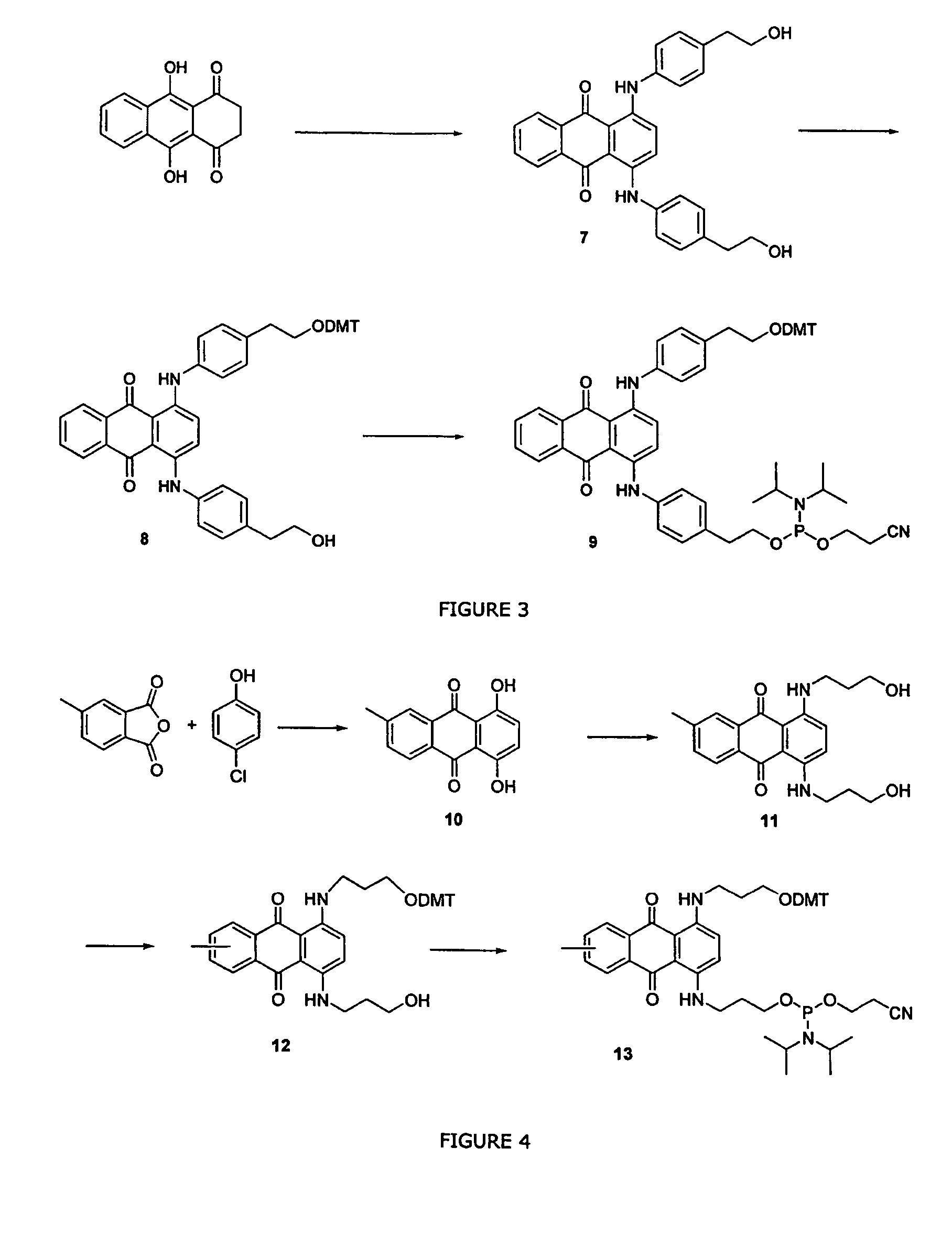
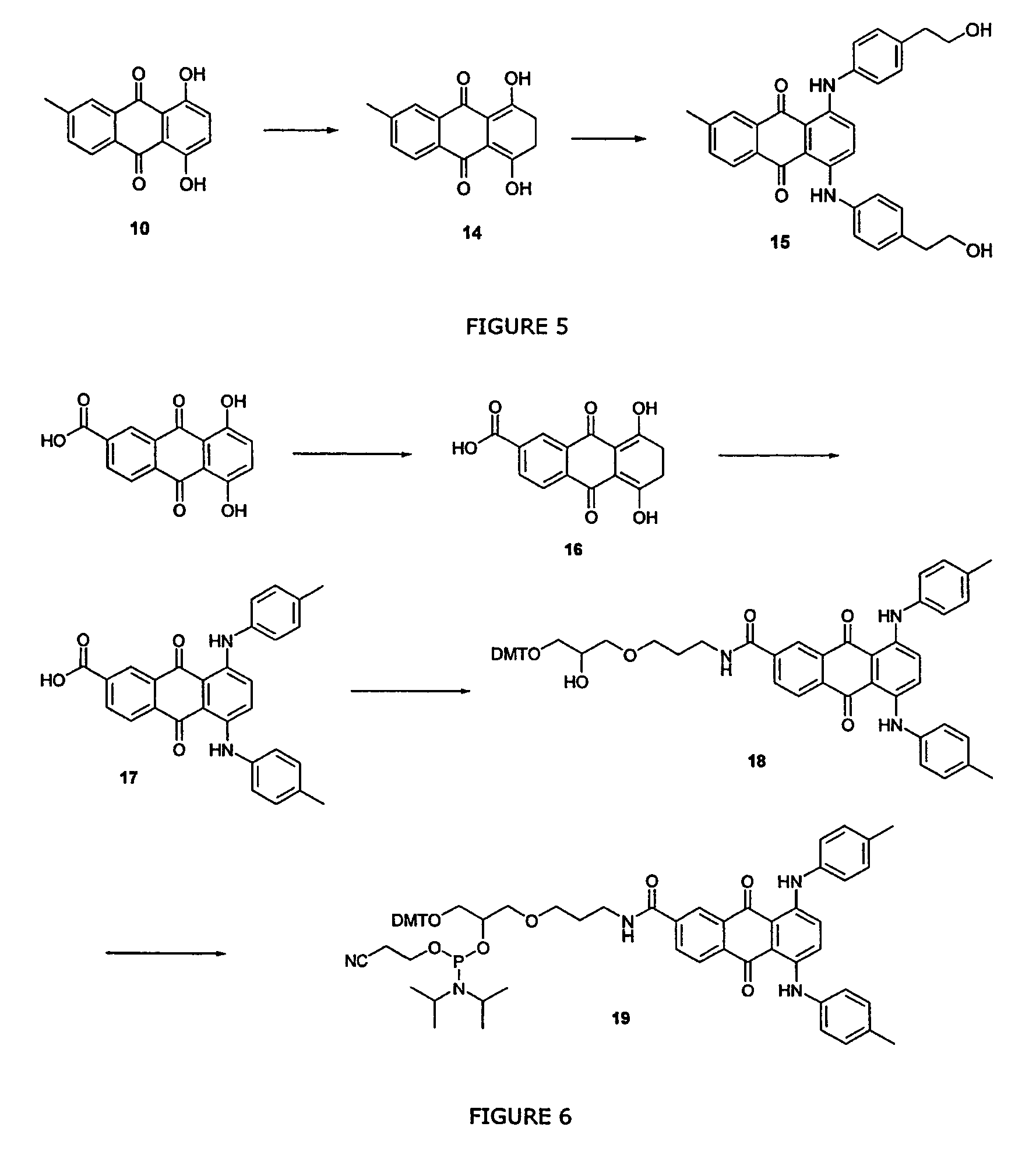
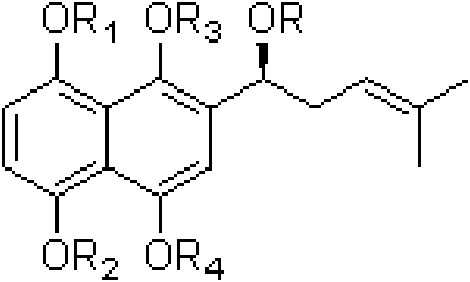
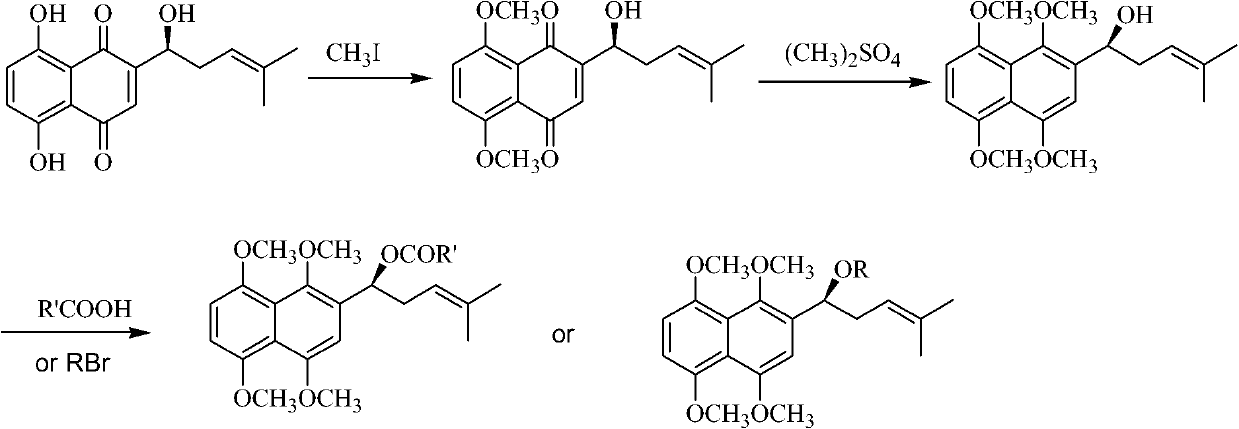
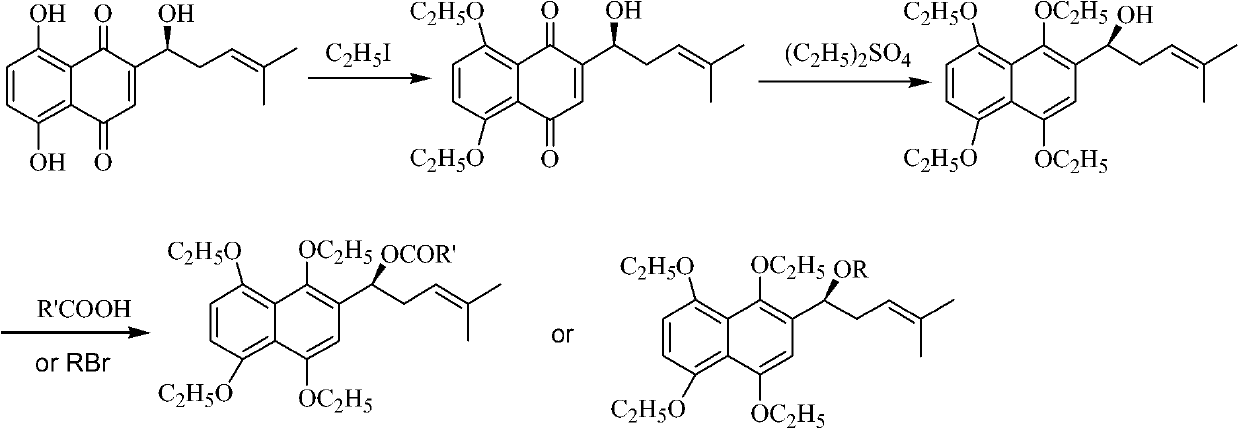
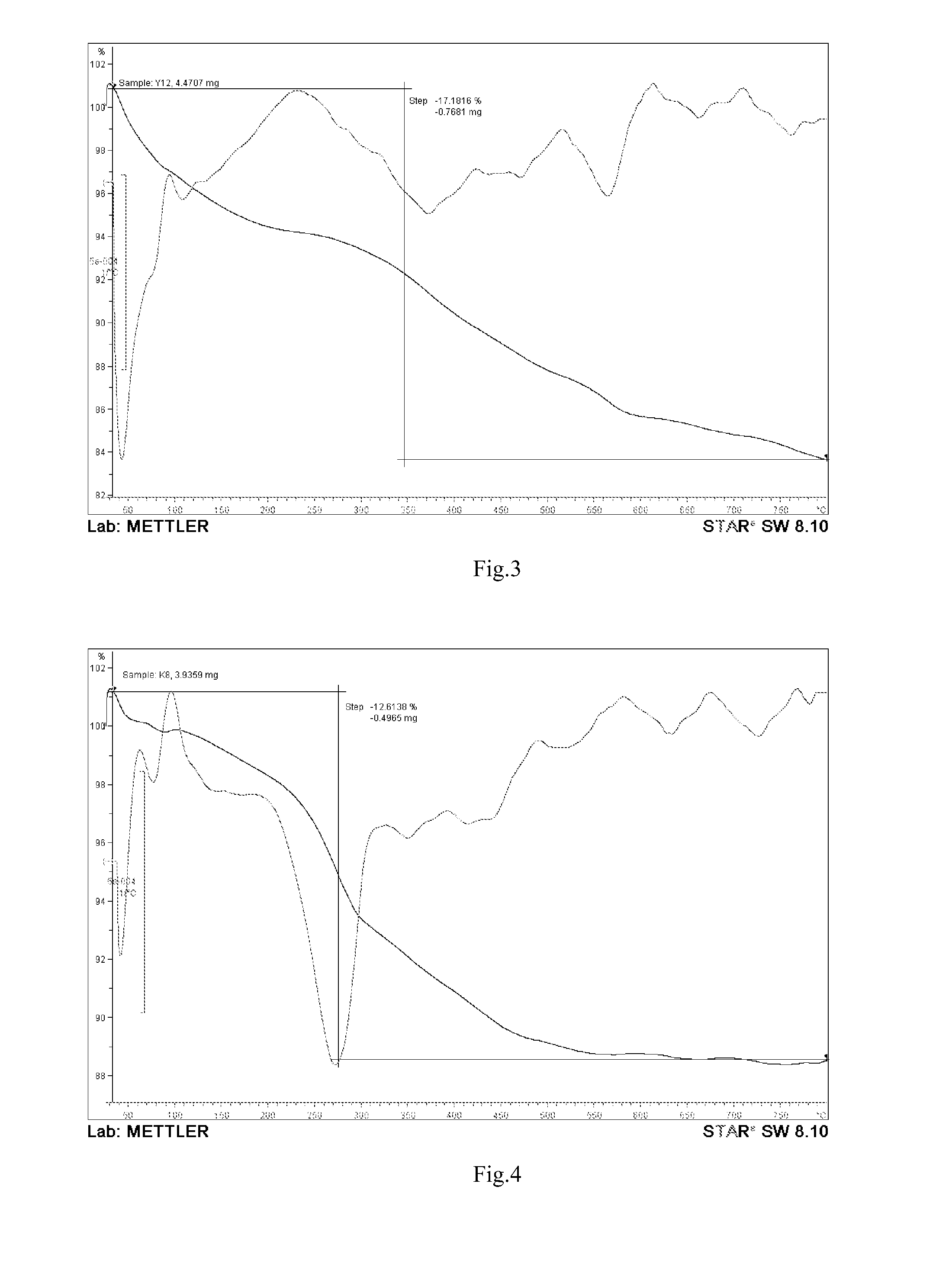
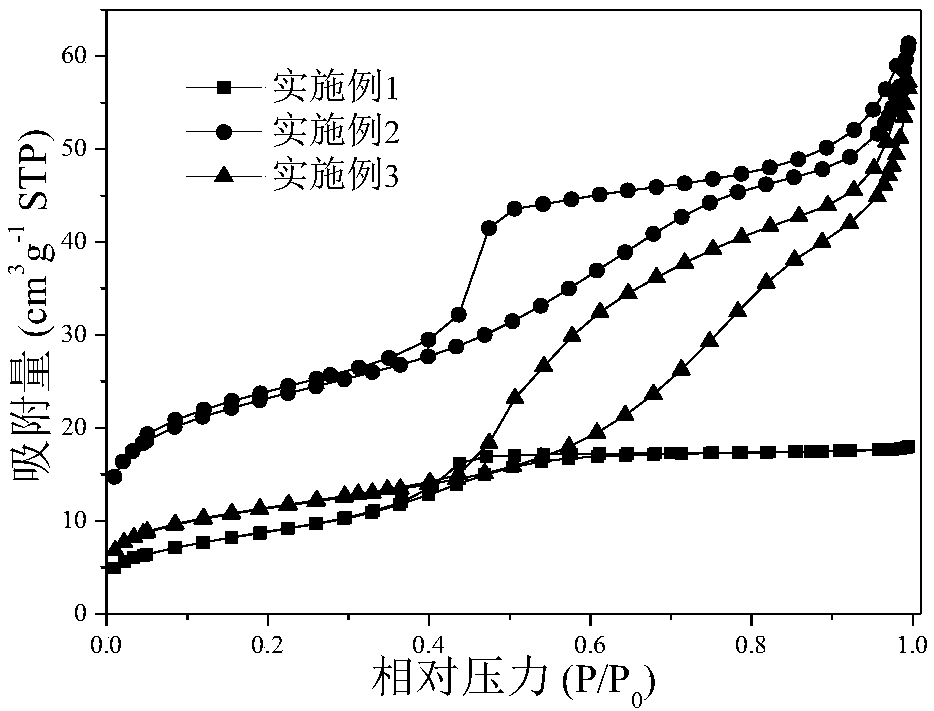
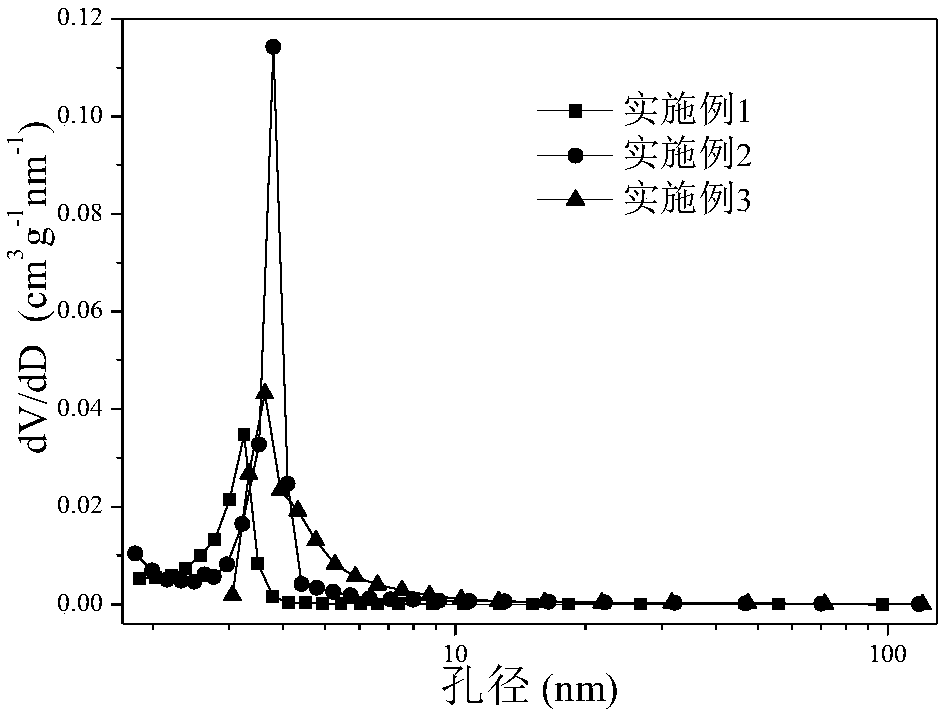
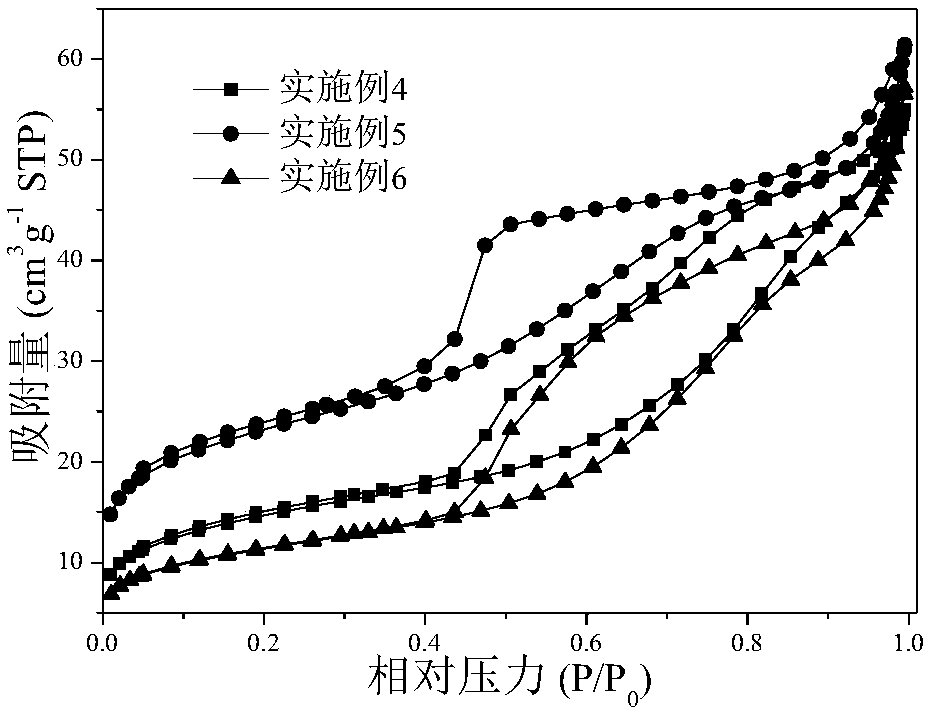
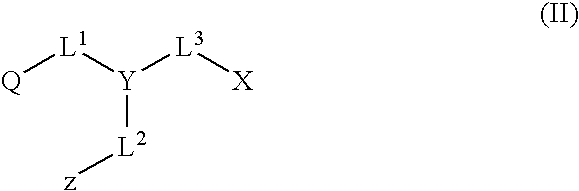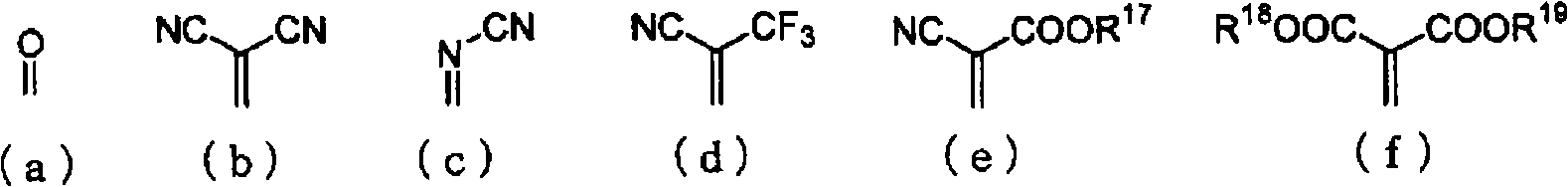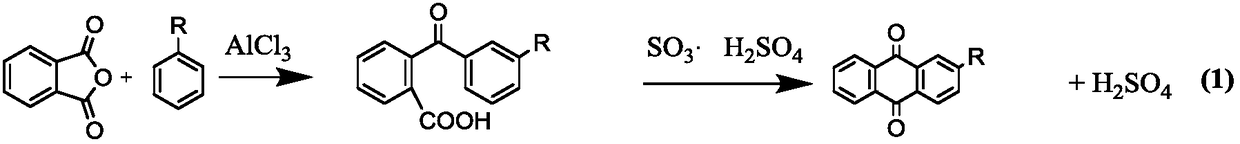Patents
Literature
344results about "Quinone preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
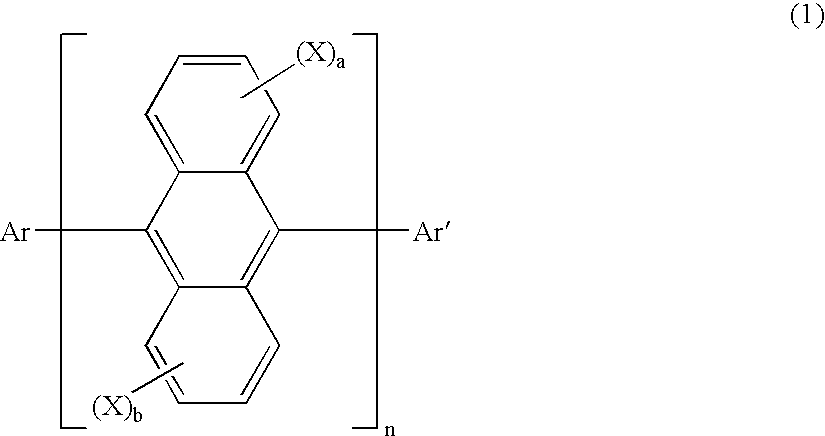
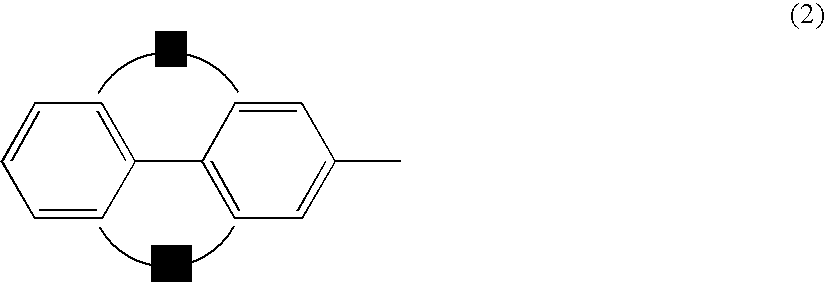
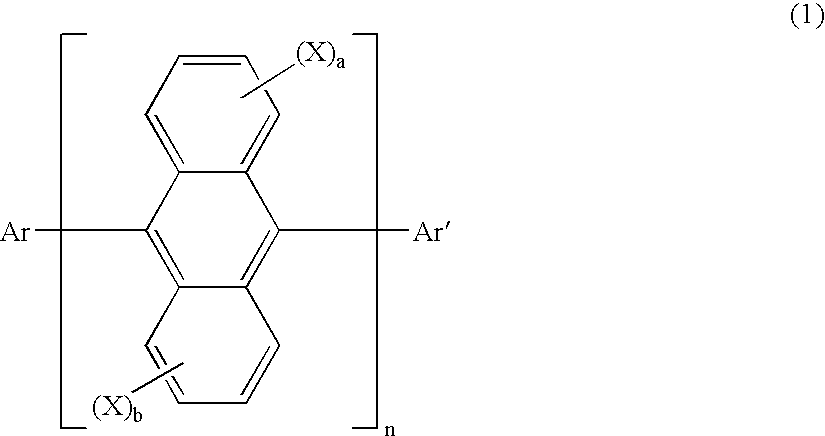
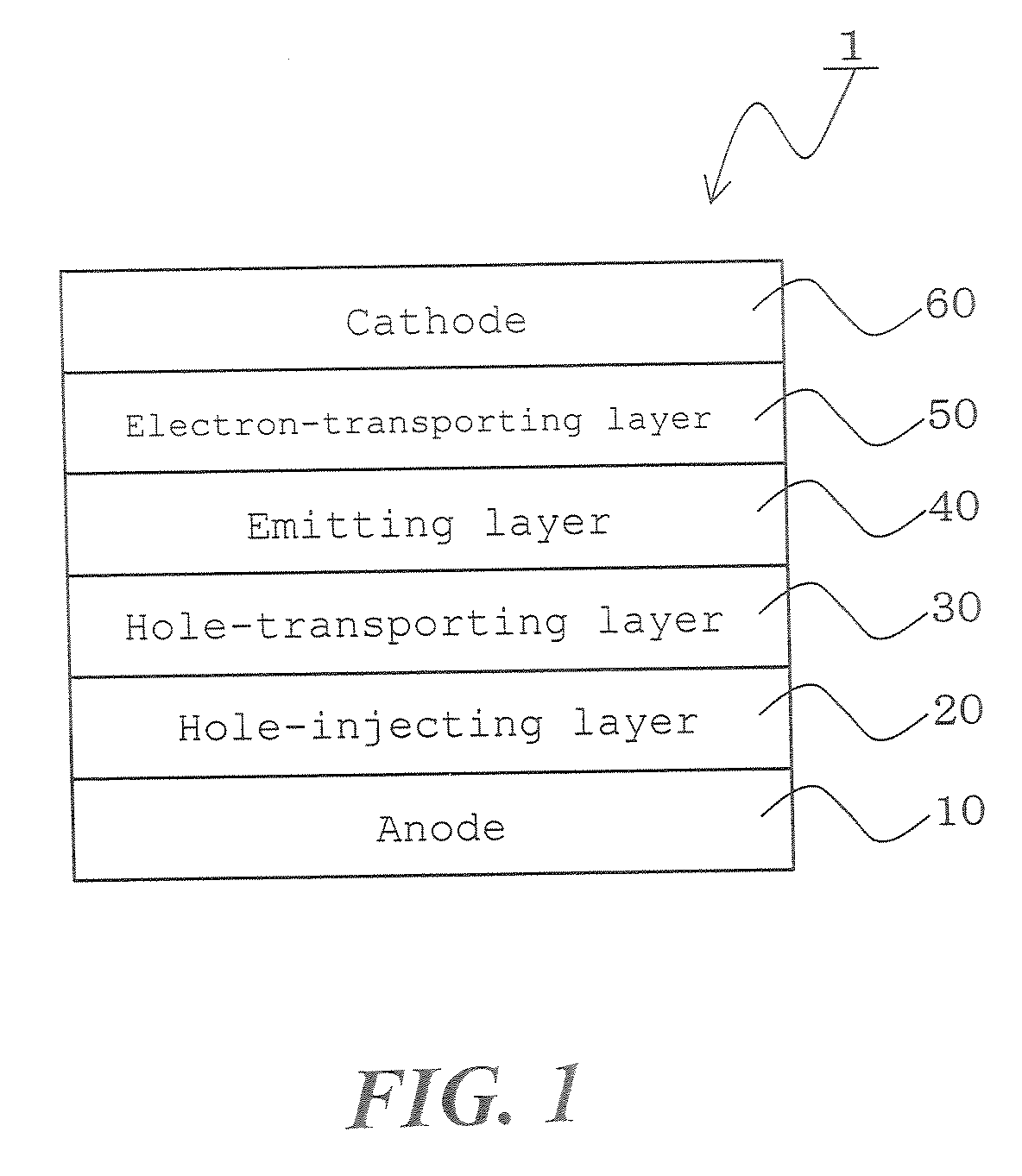
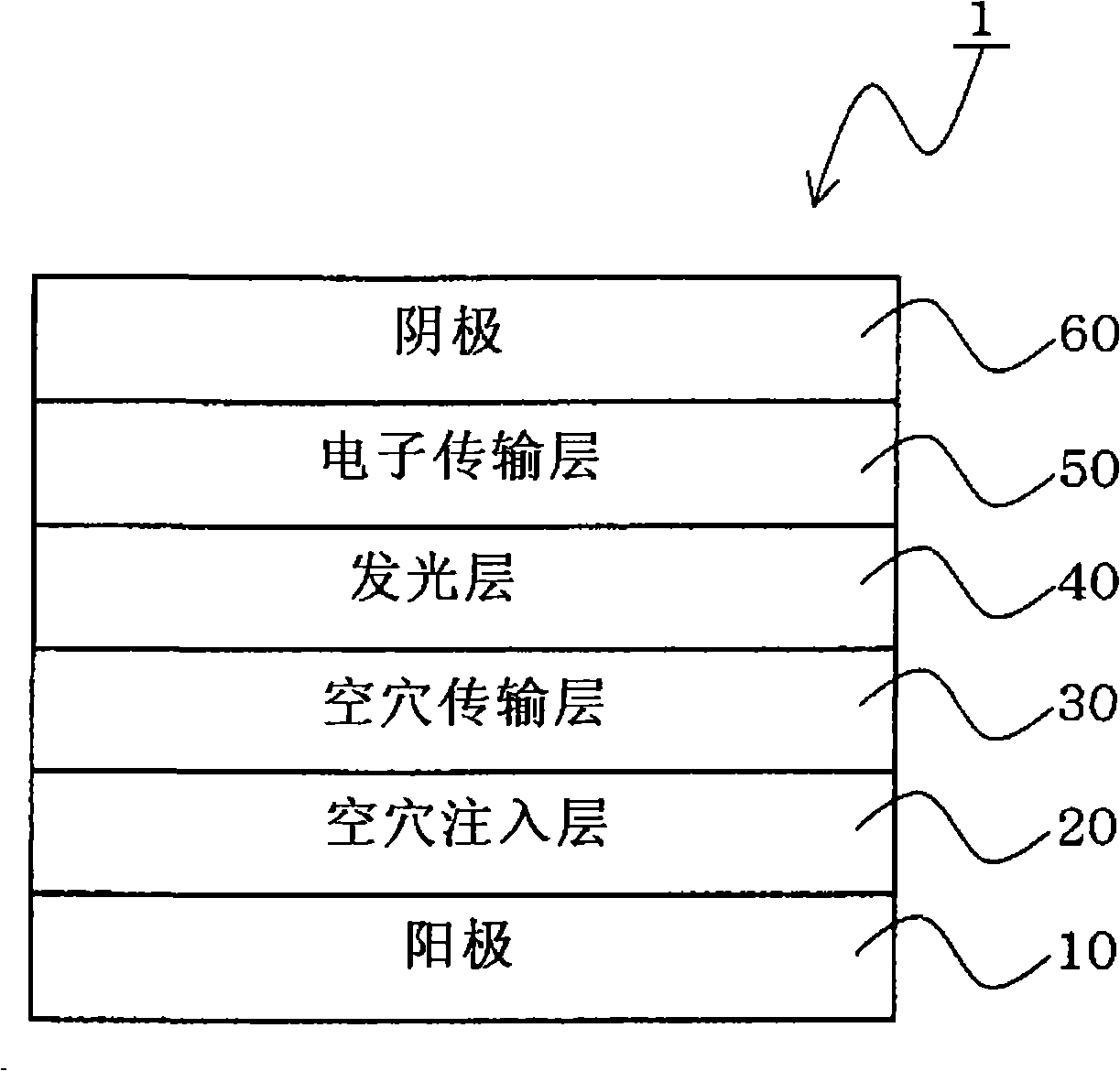
Anthracene derivatives and organic electroluminescent devices made by using the same
InactiveUS20050233165A1Improve efficiencyLight evenlySilicon organic compoundsDischarge tube luminescnet screensArylAnthracene
An anthracene derivative represented by the following general formula (1) which enables an organic electroluminescence device to exhibit a great efficiency of light emission and uniform light emission even at high temperatures since crystallization is suppressed and no thermal decomposition takes place during vapor deposition and an organic electroluminescence device utilizing the derivative, are provided. [Ar represents a group represented by the following general formula (2): (L1 and L2 each represent a substituted or unsubstituted methylene group, ethylene group or the like, and at least one of them is present), Ar′ represents a substituted or unsubstituted aryl group having 6 to 50 nuclear carbon atoms, X represent an alkyl group or the like, a and b each represent an integer of 0 to 4, and n represents an integer of 1 to 3.]
Owner:IDEMITSU KOSAN CO LTD
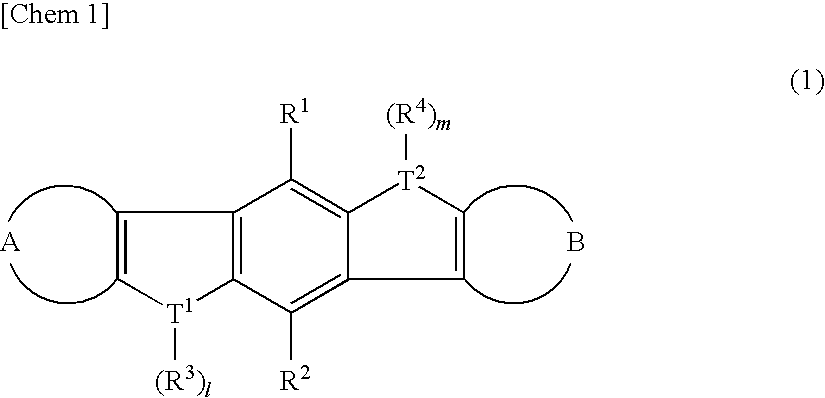
Heteroacene derivative, tetrahaloterphenyl derivative, and processes for producing the same
ActiveUS20090261300A1Improve antioxidant capacityOrganic compound preparationConductive materialOrganic filmSemiconductor materials
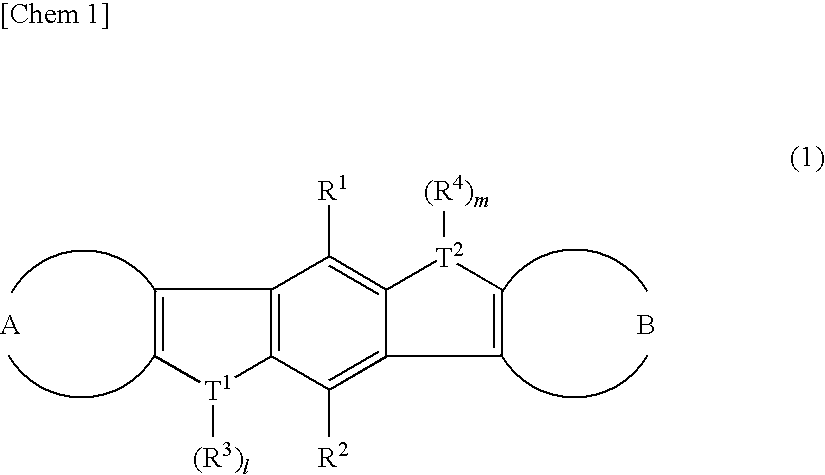
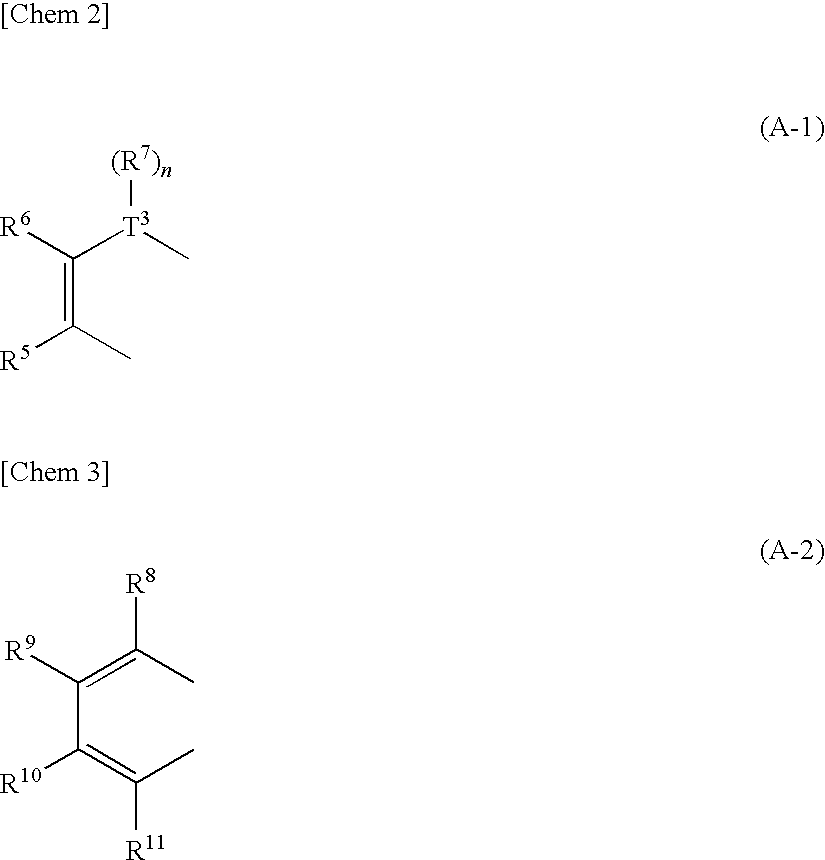
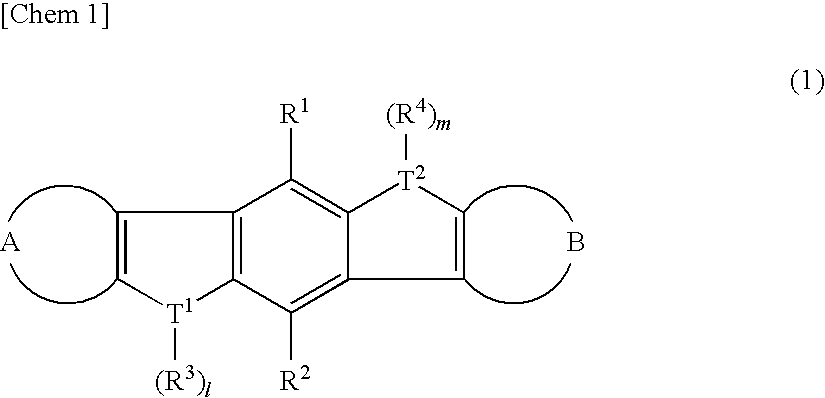
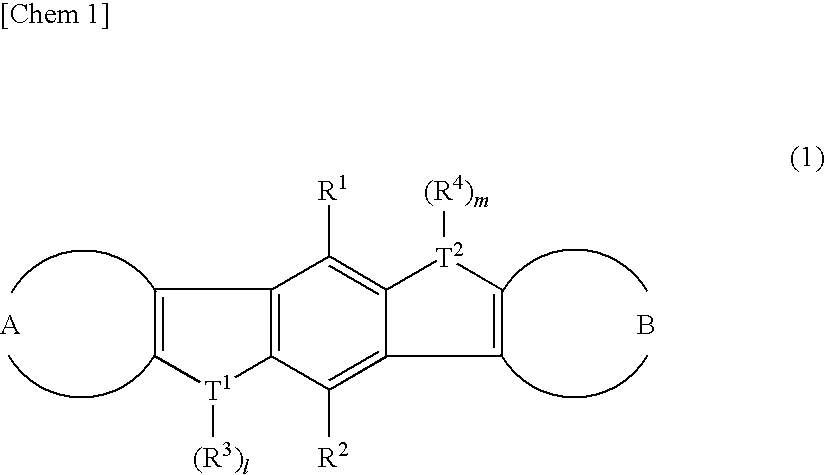
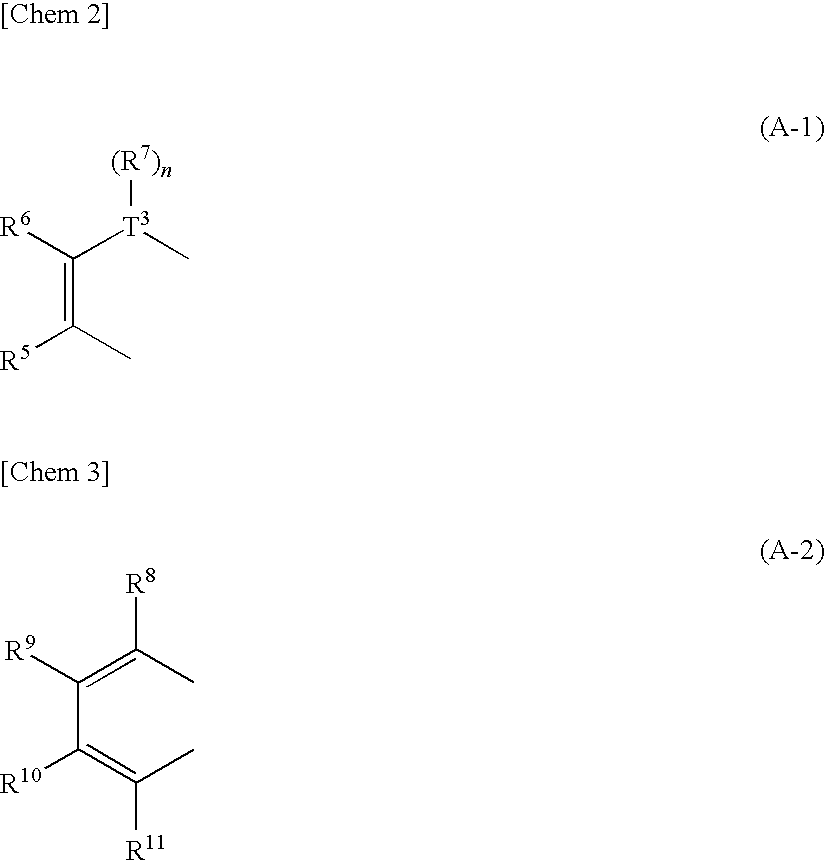
There are provided a heteroacene derivative having an excellent oxidation resistance and capable of forming a semiconductor active phase by a coating process, and an oxidation-resistant organic semiconductor material using the same, as well as an organic thin film.[Means for Resolution] A heteroacene derivative represented by the formula (1) is obtained by tetrametalation of a tetrahaloterphenyl derivative with a metalation agent and subsequent treatment of the resulting compound with reaction agents:wherein the substituents R1 to R4 are the same or different and each represents a hydrogen atom, a fluorine atom, a chlorine atom, an aryl group having 4 to 30 carbon atoms, an alkyl group having 3 to 20 carbon atoms, or a halogenated alkyl group having 1 to 20 carbon atoms; T1 and T2 are the same or different and each represents sulfur, selenium, tellurium, oxygen, phosphorus, boron, or aluminum; l and m each is an integer of 0 or 1; and rings A and B are the same or different and each has a structure represented by the following formulae (A-1) or (A-2).
Owner:TOSOH CORP
Macrocyclic and cage-like molecules based on biphenylarene and derivatives of macrocyclic and cage-like molecules as well as synthetic methods and applications of macrocyclic and cage-like molecules and derivatives
ActiveCN110642684AAchieving Modular SynthesisEasy to take offIon-exchanger regenerationOrganic chemistry methodsPorphyrinPhenyl group
The invention discloses macrocyclic and cage-like molecules based on biphenylarene and derivatives of the macrocyclic and cage-like molecules as well as synthetic methods and applications of the macrocyclic and cage-like molecules and the derivatives. A series of novel macrocycles are obtained mainly by performing a reaction on bis(2,4-dialkoxyphenyl)arenes (naphthalene, anthracene, pyrene, porphyrin and the like) or tris(2,4-dialkoxyphenyl)arenes (benzene, sym-tribenzobenzene) and paraformaldehyde under the catalysis of a Lewis acid in high yield. In addition, perhydroxybiphenylarenes (quaterphenyl trimer, naphthalene dimer and the like) can be obtained by performing demethylation, a plurality of water-soluble derivatives can be obtained by performing further modification, and the derivatives show good bonding ability to guest molecules (viologen and the like); and moreover, functional groups introduced into the framework make the biphenylarene have excellent adsorption and separationability and photophysical properties. The compounds, derivatives and methods provided by the invention have the following advantages: raw materials of the biphenylarene are commercially available, the synthesis is simple and convenient, the yield is high, and the modification is convenient, so that the compounds and the derivatives have broad application prospects in gas adsorption and separation, performance improvement of light-emitting materials, and adsorption of water-soluble toxic substances.
Owner:TIANJIN NORMAL UNIVERSITY
Quencher compositions comprising anthraquinone moieties
The present invention provides novel quencher composition comprising anthraquinone quencher moieties. The anthraquinone quencher moieties are useful as quencher labels when attached to biomolecules such as natural or modified polynucleotides, oligonucleotides, nucleosides, nucleotides, carbohydrates and peptides. For example, polynucleotides can be labeled at the 3′ terminus with fluorescence quencher solid support compositions, and polynucleotides can be labeled at internally or at the 5′ terminus. The detectable probes may have a format like molecular beacons, scorpion probes, sunrise probes, conformationally assisted probes and TaqMan probes.
Owner:QIAGEN GMBH
Synthesis method of 2-alkyl anthraquinone
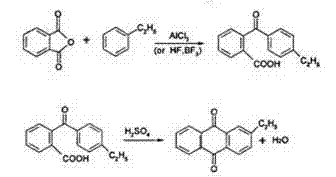
ActiveCN1651386ASimple processEfficient processOrganic compound preparationQuinone preparationBenzoic acidSynthesis methods
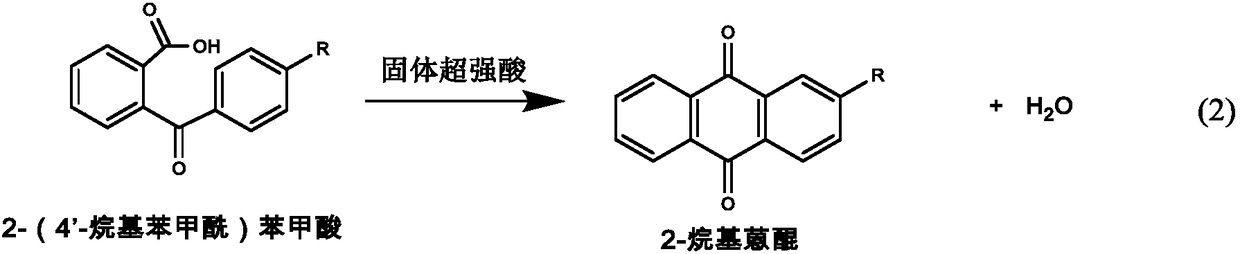
A process for preparing 2-alkylanthraquinone from 2-(4'-alkylbenzoyl) benzoic acid includes such steps as mixing said raw material with solid acid catalyst and closed-loop dewatering reacting. Its advantages are high purity, high transform rate and high selectivity.
Owner:DALIAN UNIV OF TECH
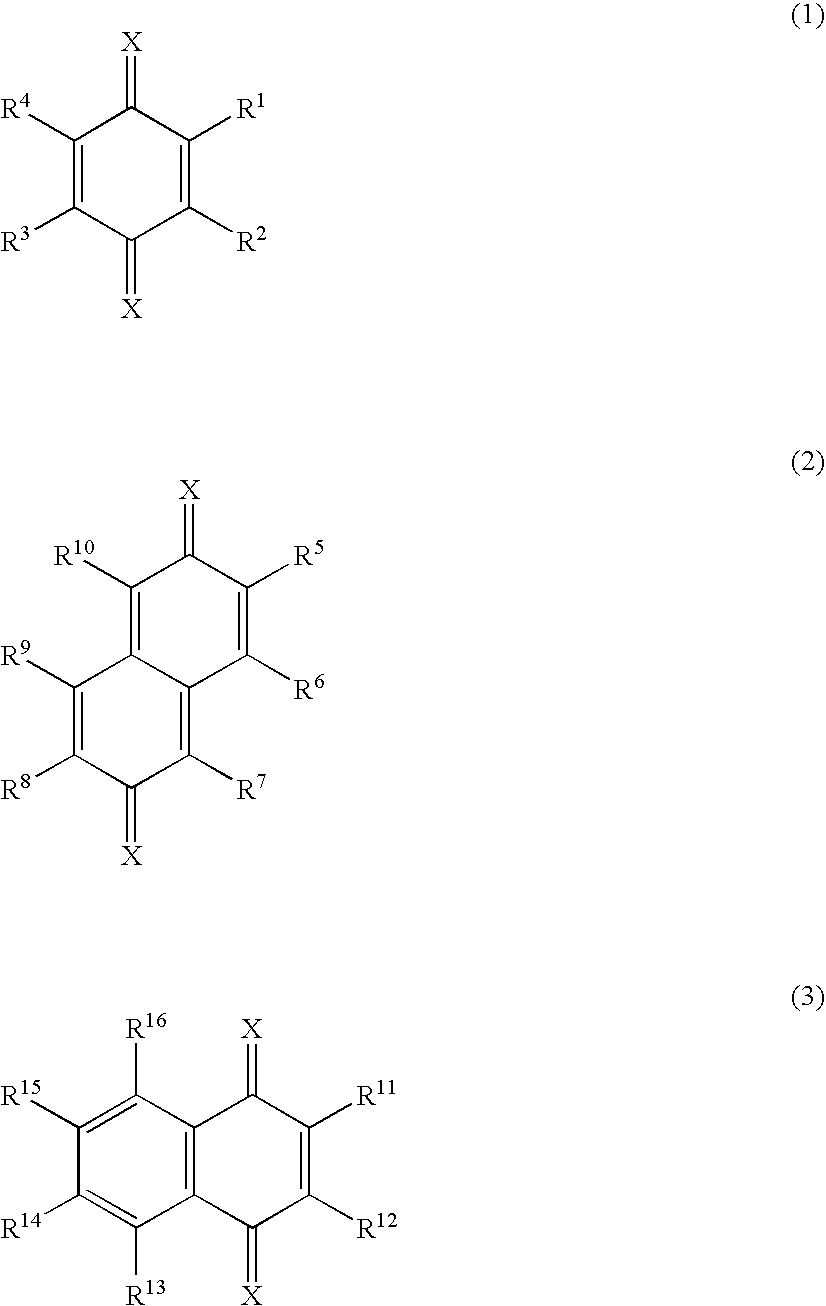
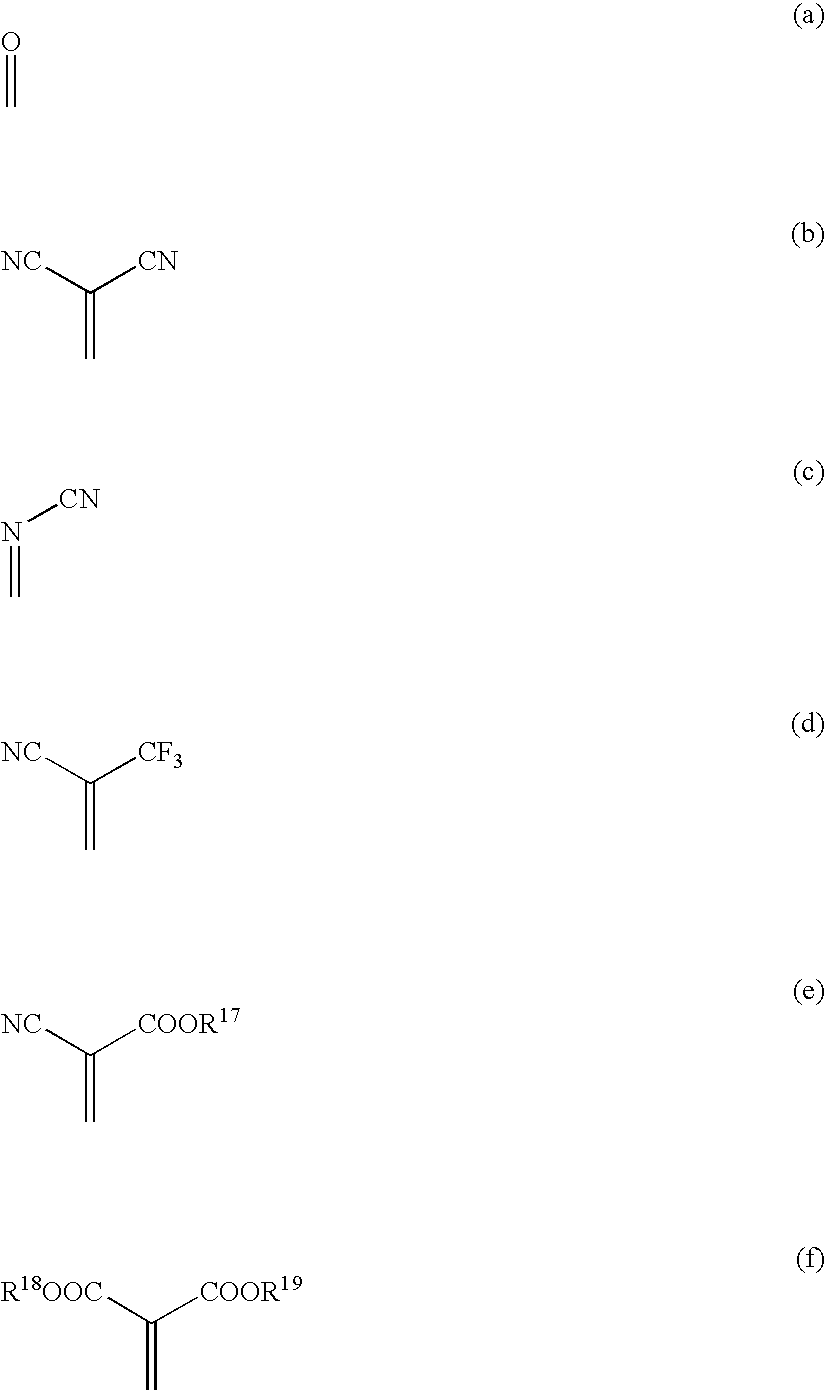
Material for organic electroluminescent device and organic electroluminescent device using the same
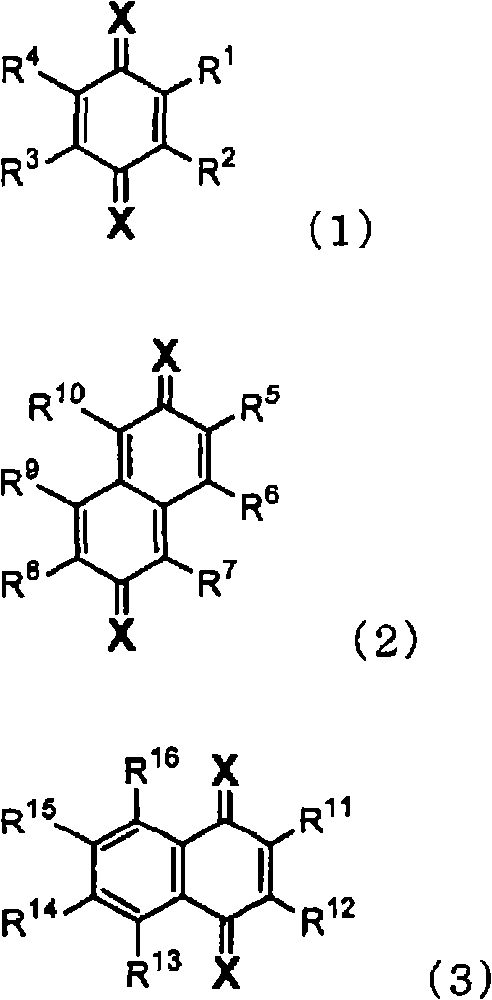
InactiveUS20080093985A1High propertyImproved heat resistance and crystallizing suppressionDischarge tube luminescnet screensOrganic compound preparationQuinoneAryl
A material for an organic electroluminescent device including a quinone derivative represented by the following formula (1), (2) or (3): wherein R1 to R16 are each a hydrogen atom, a halogen atom, a cyano group, an alkoxy group, a substituted or unsubstituted aryloxy group, an alkyl group, a fluoroalkyl group, an aryl group or a heterocyclic group; provided that at least one of R1 to R4, at least one of R5 to R10 or at least one of R11 to R16 is an aryloxy group; and X is a substituent represented by any one of the following formulas (a) to (f): wherein R17 to R19 are a hydrogen atom, an alkyl group, or aryl group; and R18 and R19 may be bonded together to form a ring.
Owner:IDEMITSU KOSAN CO LTD
Method for catalytically preparing 2-ethyl anthraquinone by alkali desilicicated modified Hbeta molecular sieve
ActiveCN103833534AEasy to separateEasy to operateMolecular sieve catalystsOrganic compound preparationBenzoic acidMolecular sieve
The invention discloses a method for catalytically preparing 2-ethyl anthraquinone by an alkali desilicicated modified Hbeta molecular sieve, and relates to a method for preparing 2-ethyl anthraquinone by dehydration of 2-(4'-ethyl benzoyl) benzoic acid. The method provided by the invention aims to solve the technical problem that by adopting a concentrated sulfuric acid catalyst to industrially produce 2-ethyl anthraquinone in the prior art, a lot of acid wastewater is generated and the equipment is greatly corroded. The method comprises the following steps: I, preparing an alkali desilicicated modified Hbeta molecular sieve catalyst; and II, reacting to prepare 2-ethyl anthraquinone. The catalyst Hbeta molecular sieve used in the method is environment-friendly solid acid, the acidity and mesoporous dimension of which can be conveniently adjusted by changing conditions of alkali treatment. The process for preparing 2-ethyl anthraquinone belongs to a heterogeneous catalytic process which is simple to operate, and the catalyst after reaction can be conveniently separated, so that the alkali desilicicated modified Hbeta molecular sieve catalyst is high in activity, recyclable and free from any wastewater, so that the method is an environment-friendly method for preparing 2-ethyl anthraquinone.
Owner:宜兴利荣达科技有限公司
Heteroacene derivative, tetrahaloterphenyl derivative, and processes for producing the same
ActiveUS8138355B2Improve antioxidant capacityOrganic compound preparationGroup 5/15 element organic compoundsSemiconductor materialsOxidation resistant
There are provided a heteroacene derivative having an excellent oxidation resistance and capable of forming a semiconductor active phase by a coating process, and an oxidation-resistant organic semiconductor material using the same, as well as an organic thin film.A heteroacene derivative represented by the formula (1) is obtained by tetrametalation of a tetrahaloterphenyl derivative with a metalation agent and subsequent treatment of the resulting compound with reaction agents:wherein the substituents R1 to R4 are the same or different and each represents a hydrogen atom, a fluorine atom, a chlorine atom, an aryl group having 4 to 30 carbon atoms, an alkyl group having 3 to 20 carbon atoms, or a halogenated alkyl group having 1 to 20 carbon atoms; T1 and T2 are the same or different and each represents sulfur, selenium, tellurium, oxygen, phosphorus, boron, or aluminum; l and m each is an integer of 0 or 1; and rings A and B are the same or different and each has a structure represented by the following formulae (A-1) or (A-2).
Owner:TOSOH CORP
Anthraquinone derivative material and preparation method and application thereof
ActiveCN102795983AImprove thermal stabilityImprove structural stabilityOrganic compound preparationSolid-state devicesAnthraquinone DerivativesThermal stability
The invention discloses an anthraquinone derivative material, which has the following structural formula: wherein -Ar is FORMULA. The anthraquinone derivative material has excellent thermal stability and conjugate structure, and emits blue light. The invention also provides a preparation method for the anthraquinone derivative material and application of the material in organic electroluminescent devices.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Anthraquinone degradation product regeneration catalyst and preparation method thereof
InactiveCN102728338AReduce manufacturing costSolution to short lifeOrganic compound preparationQuinone preparationHydrotalciteCompressive strength
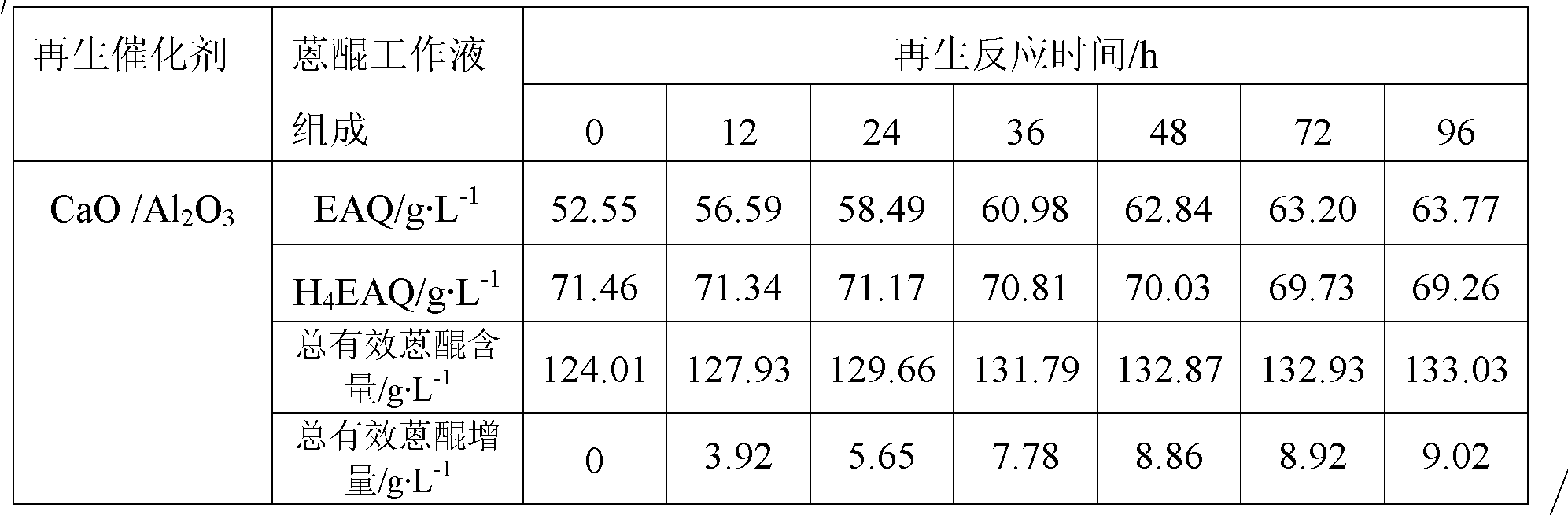
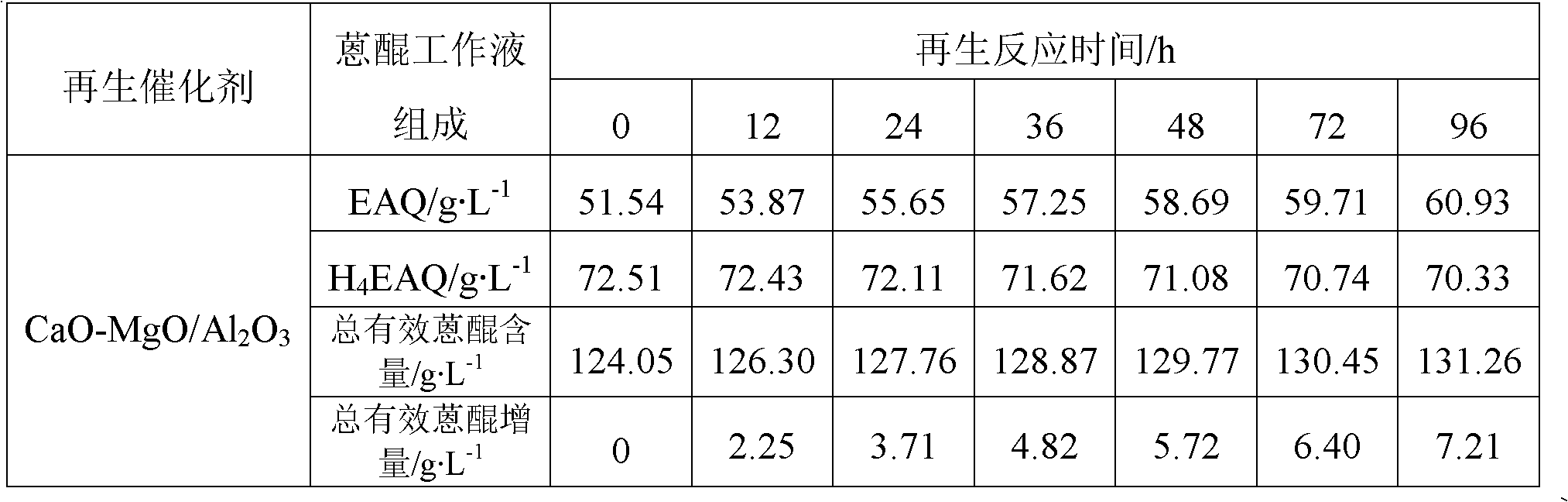
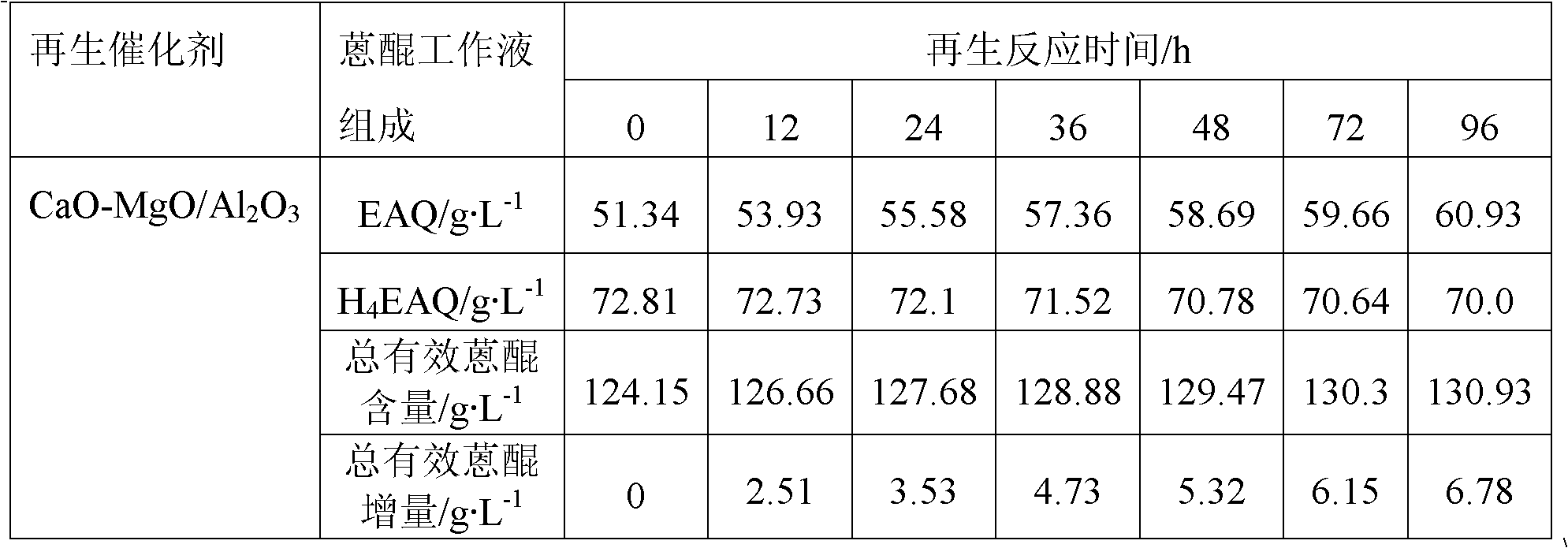
The invention provides an anthraquinone degradation product regeneration catalyst and a preparation method thereof. MeO / Al2O3 represents the anthraquinone degradation product regeneration catalyst, wherein MeO represents an alkaline oxide; a crystal form of Al2O3 is a gamma form; Al2O3 crystals are in shapes of balls, strips and whitetip clover; a mass percent of MeO is in a range of 1 to 60wt%; a specific surface area of MeO / Al2O3 is in a range of 100 to 300m<2>.g<-1>; a pore volume is in a range of 0.3 to 1.5cm<3>.g<-1>; and compressive strength is in a range of 50 to 150N / particle. The preparation method comprises the following steps of synthesizing a hydrotalcite MeAlCO3-LDH / Al2O3 precursor on surfaces of Al2O3 carriers and in channels of the Al2O3 carriers and carrying out calcination at a temperature of 500 to 600 DEG C to obtain the anthraquinone degradation product regeneration catalyst. The anthraquinone degradation product regeneration catalyst which is an alkaline composite oxide can be used for regeneration of an anthraquinone working solution used in an anthraquinone process-based hydrogen peroxide preparation industry and has a stable regeneration period of 6 to 20 months. Compared with the existing regeneration catalyst used industrially, the anthraquinone degradation product regeneration catalyst has a service life prolonged by 2-3times and higher regeneration efficiency.
Owner:BEIJING UNIV OF CHEM TECH
Device and process for continuously producing 2-ethyl anthracene quinone in channelization way
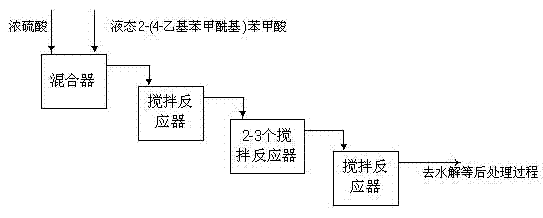
InactiveCN101633612AQuick mixForced flowOrganic compound preparationQuinone preparationBenzoic acidQuinone
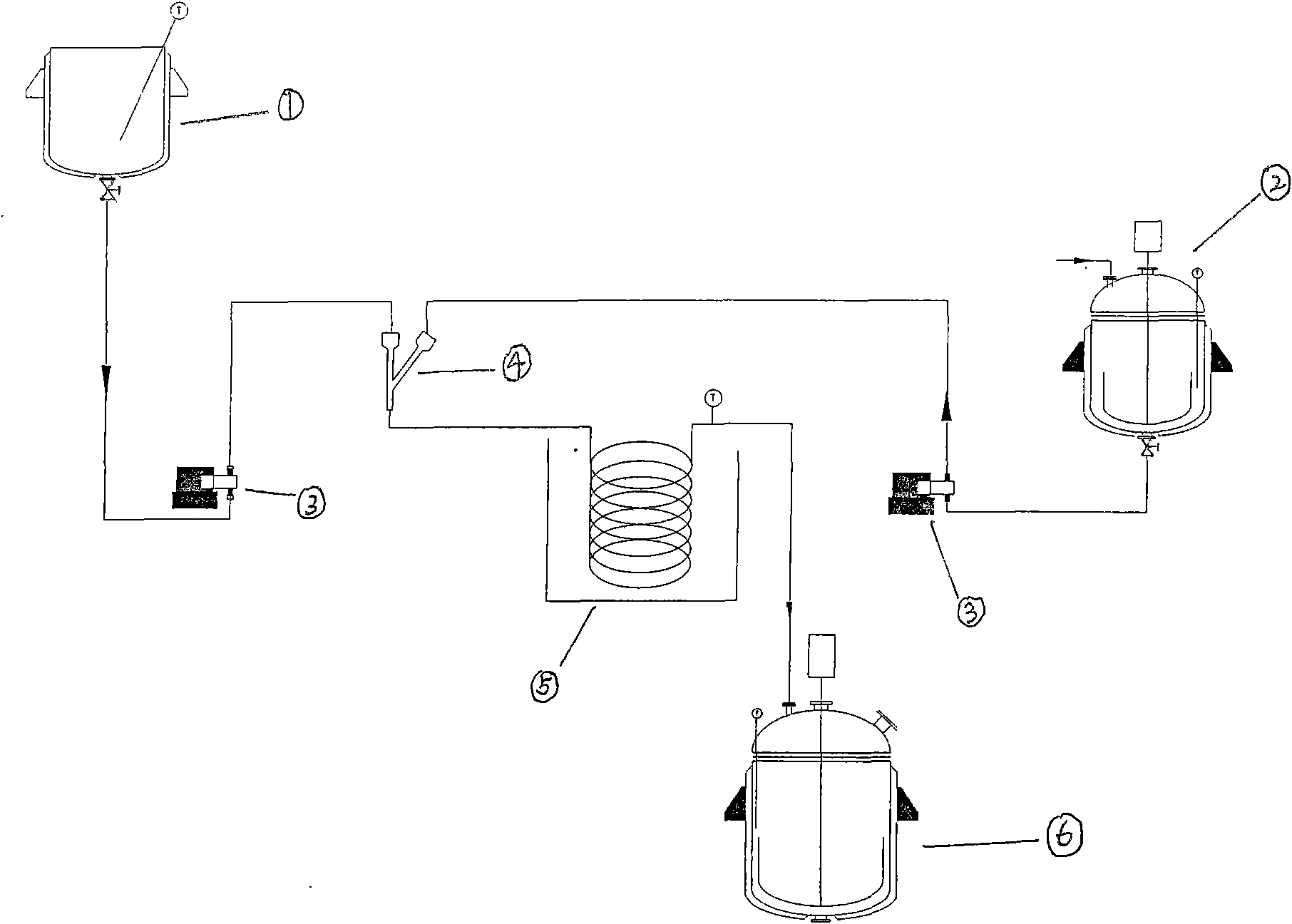
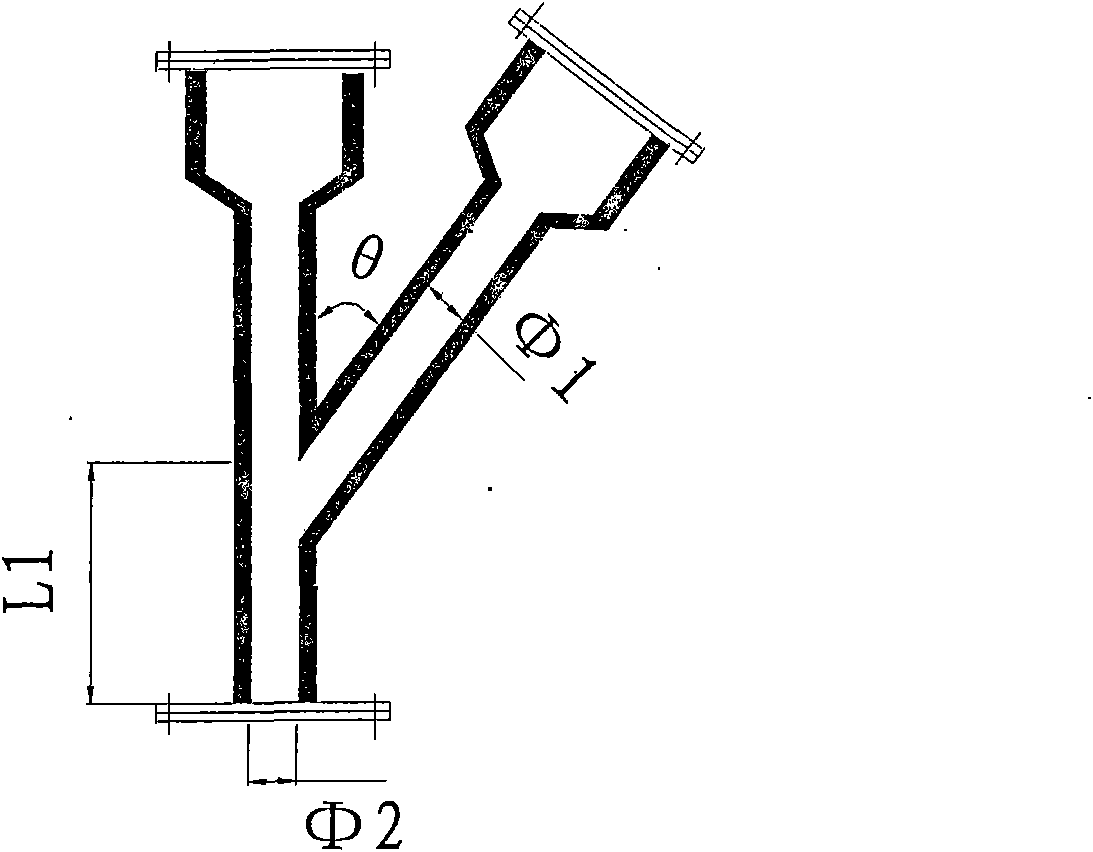
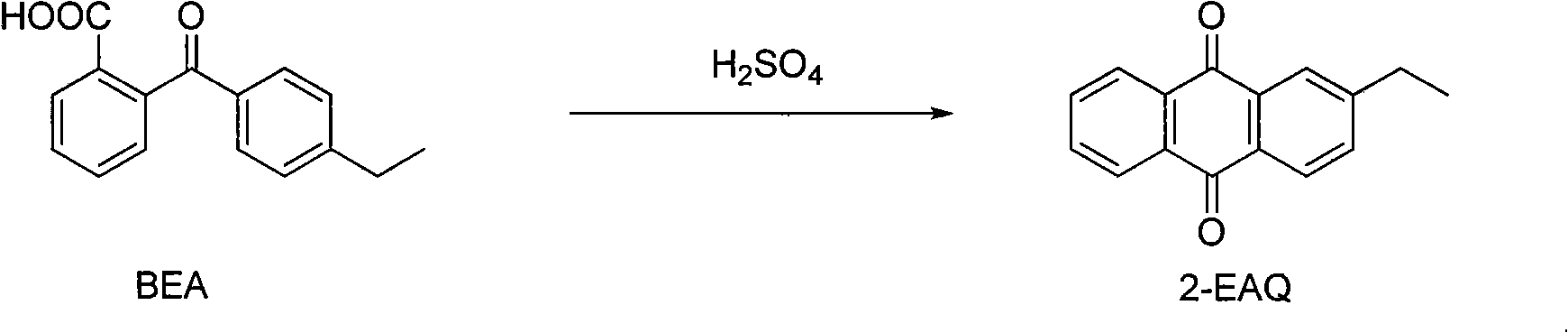
The invention discloses a device and a process for continuously producing 2-ethyl anthracene quinone in a channelization way. The device is shown in the figure, and the process is specifically shown as below: putting fuming sulfuric acid in a second storage tank; putting solid raw material 2-(4- ethyl benzene formoxyl) benzoic acid in a storage tank I; rising the temperature to 125-190 DEG C to keep the 2-(4- ethyl benzene formoxyl) benzoic acid in a liquid state; rapidly and evenly mixing the 2-(4- ethyl benzene formoxyl) benzoic acid in the storage tank I and the fuming sulfuric acid in a storage tank II in a Y-type jet mixer; and then filling the mixture in a pipe-type reactor, controlling the reaction temperature in the pipe-type to be 120-180 DEG C and the reaction time to be 2-10 min; and hydrolyzing the obtained reaction liquid in a hydrolization pot to separate out the product 2-ethyl anthracene quinone. The device has the advantages of simplicity, high process safety, easy control of reaction conditions, realization of serialization production, productivity reaching more than 90 percent, stable product quality and large scale industrial production by only less investment.
Owner:ZHEJIANG JINKE CHEM
Method for synthesizing producets in vitamin K2 series
ActiveCN101092340AReduce isomerizationReduced responseOrganic compound preparationQuinone preparationVitamin K2Organic synthesis
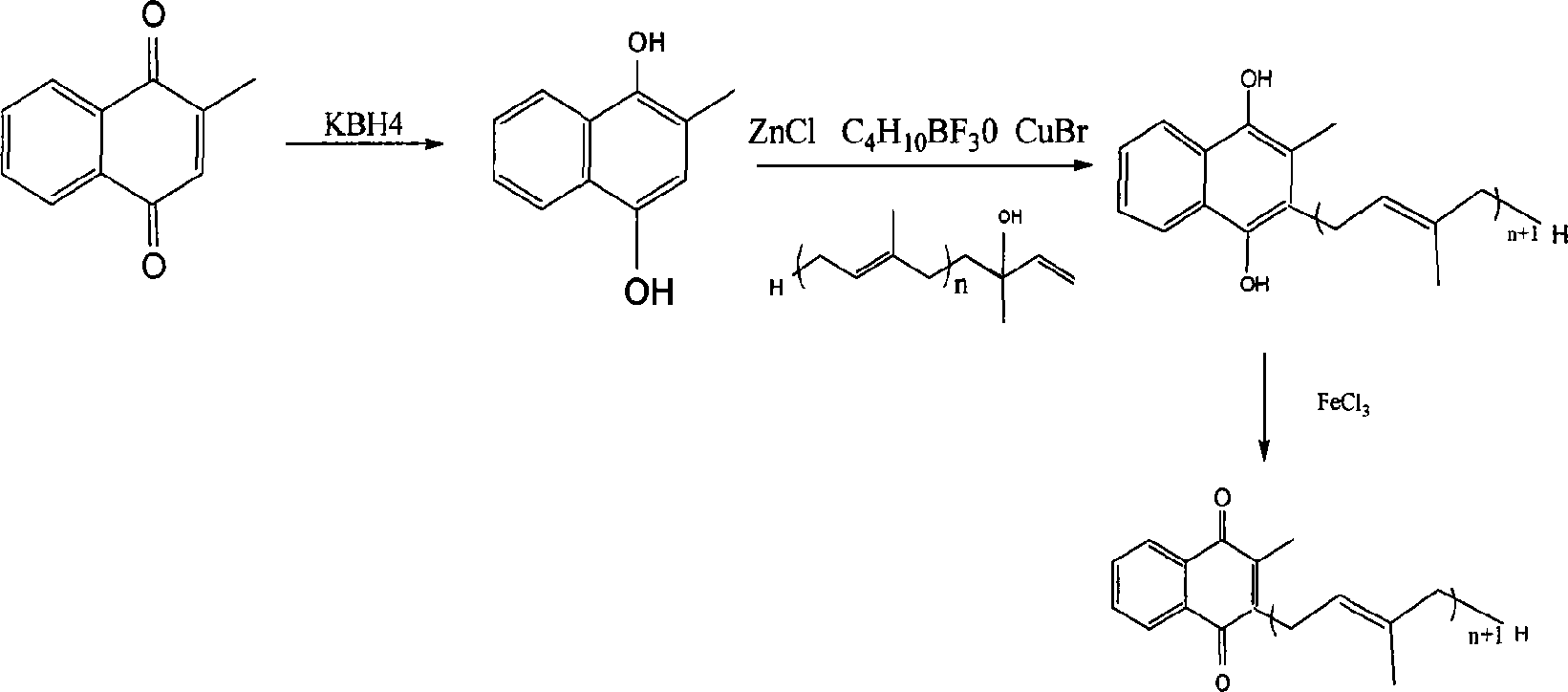
This invention discloses a method for synthesizing vitamin K2 product. The method comprises: reducing 2-methyl-1, 4-naphthoquinone into hydroquinone by using KBH4, performing Fridel-Crafts alkylation reaction with long-chain tert-terpenol in nitromethane / n-hexane system with anhydrous ZnCl2, boron trifluoride diethyl etherate and CuBr as the catalysts to introduce long-chain terpenyl to the C3 site of hydroquinone, oxidizing with isopropanol solution of FeCl3 to obtain corresponding vitamin K2. The method has such advantages as mild reaction conditions, high yield, recoverable reagents, and no environmental pollution.
Owner:广东固升医药科技有限公司
Method for preparing anthraquinone
ActiveCN109503348AHigh yieldReduce pollutionOrganic compound preparationQuinone preparationSalt waterTurbidity
The invention discloses a method for preparing anthraquinone. The method comprises procedures of condensation, hydrolysis, cycle closure, dilution procedures, mother liquid retreatment procedures andwaste sulfuric acid retreatment procedures. A simple process is adopted, circulation is achieved, waste is turned into wealth, commercial chemical raw materials are respectively recycled from waste aluminum salt water and waste sulfuric acid water, the comprehensive preparation cost is greatly reduced, and the environment pollution is reduced. Wastewater with an aluminum salt is generated in the hydrolysis procedure, that is, a mother liquid, the mother liquid contains a certain amount of o-benzoylbenzoic acid, after improvement of the hydrolysis procedure, unseparated o-benzoylbenzoic acid ina lower layer of the mother liquid can be separated, the yield of the anthraquinone can be increased, the purity of a composite water purifier in the mother liquid can be improved, and the compositewater purifier is free of impure color. In addition, the turbidity removal capability of the composite water purifier prepared by using the method is remarkably improved.
Owner:辽宁坤泰化工有限公司
Functionalized column aromatic hydrocarbon derivative and preparation method thereof
ActiveCN109400501AThe means of expanding functionalityAddresses less functional flawsOrganic compound preparationSulfonic acid esters preparationAromatic hydrocarbonOxime
The invention discloses a functionalized column aromatic hydrocarbon derivative and a preparation method thereof. The functionalized column aromatic hydrocarbon derivative comprises an X unit, a Y unit and a Z unit, wherein the X unit is shown in a formula (I), the Y unit is shown in a formula (II), the Z unit is shown in a formula (III), and the six-membered rings of the X unit, the Y unit and the Z unit all have two para-position substituents and four unsubstituted positions; the formulas are as shown in the specification, each unit is connected through CH2 groups to form a ring-shaped structure, and each unit is connected with the other two adjacent units in a para-position manner at an unsubstituted position; and the formed ring-shaped structure is the functionalized column aromatic hydrocarbon derivative. Cyano groups, carboxyl groups and oxime are directly connected to the benzene ring monomer of the column aromatic hydrocarbon, so that the method for functionalizing the column aromatic hydrocarbon is expanded; and the connected groups can effectively change the electronic environment of the column aromatic hydrocarbon rings, so that the functionalized column aromatic hydrocarbon derivative has wide application in the fields of supramolecular host-guest identification and the like.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Emodin derivative and application thereof in preparing antibacterial agents
InactiveCN102775291AGood anti-drug resistant bacteriaGood effectAntibacterial agentsOrganic active ingredientsStructural formulaHigh activity
The invention relates to an emodin derivative and application thereof in preparing antibacterial agents. The chemical structural formula of the emodin derivative is represented as formula (I), wherein R1, R2, R3 and R4 are -F, -Cl, -Br, -I or -NO2; and R5, R6 and R7 are -H, -CH3, -CH2CH3 and -COCH3. The emodin derivative is a high-activity compound designed by performing structural modification on compounds with natural emodin as a matrix according to the principle of medical molecules. Experiments prove that the emodin derivative has good effects of resisting drug-resistance bacteria and superbacteria, the pharmaceutical effect of the emodin derivative is remarkably better than that of the emodin, and a new choice is provided for clinical medication.
Owner:SICHUAN UNIV
Process for hydrogenation of alkyl anthraquinone by using magnetically stabilized bed
ActiveCN1690035AReduce carry outImprove mass transfer efficiencyOrganic compound preparationPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesReaction temperatureFixed bed
The alkyl anthraquinone hydrogenating reduction process is that inside magnetically stabilized bed reactor, alkyl anthraquinone solution, hydrogen and ferromagnetic hydrogenating catalyst are contacted at the conditions including reaction temperature of 25-150 deg.c, reaction pressure of 0.1-2.0 MPa, liquid spatial velocity of 3-40 / hr, hydrogen / liquid phase material feeding volume ratio of 5-300 and magnetic field strength of 1000 Oe. The ferromagnetic hydrogenating catalyst is selected from non-crystalline nickel alloy, Raney nickel or magnetic carrier loaded noble metal catalyst. Compared with fixed bed process, the process of the present invention has the advantages of lower bed pressure drop, higher mass transfer efficiency, less side reactions and less anthraquinone degrading amount. Compared with fluidized bed process, the process of the present invention has higher mass transfer efficiency and less catalyst carrying out.
Owner:CHINA PETROLEUM & CHEM CORP +1
Quencher compositions comprising anthraquinone moieties
The present invention provides novel quencher composition comprising anthraquinone quencher moieties. The anthraquinone quencher moieties are useful as quencher labels when attached to biomolecules such as natural or modified polynucleotides, oligonucleotides, nucleosides, nucleotides, carbohydrates and peptides. For example, polynucleotides can be labeled at the 3′ terminus with fluorescence quencher solid support compositions, and polynucleotides can be labeled at internally or at the 5′ terminus. The detectable probes may have a format like molecular beacons, scorpion probes, sunrise probes, conformationally assisted probes and TaqMan probes.
Owner:QIAGEN GMBH
Novel quinone compound and preparation method thereof
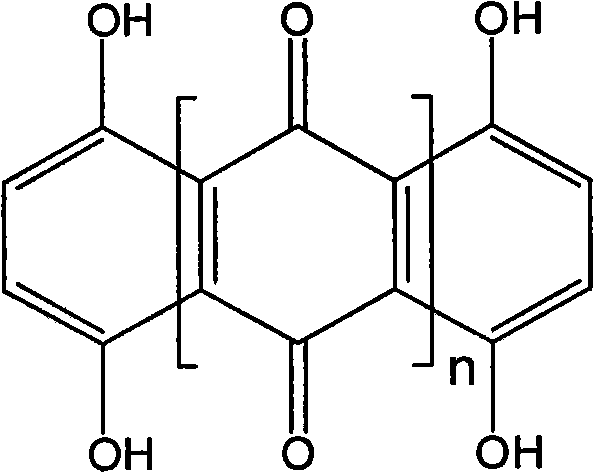
The invention relates to a novel quinone compound and a preparation method thereof. The compound has a structural formula which is shown in the specification, wherein n is equal to 4-28; and 1,4,5,8-tetrahydroxy-9,10-anthraquinone is used as a raw material and is subjected to one-step reaction in a solvent in the presence of a catalyst to prepare polyquinone oligomer. The oligomer can be used for a positive material of a high-specific-energy battery and can also be used for a photoelectric device material. The preparation method is simple and practical; a pure product can be obtained without purification; and the yield reaches over 80 percent.
Owner:NO 63971 TROOPS PLA
Method for synthesizing hypericin
ActiveCN102180782AHigh yieldShort reaction pathOrganic compound preparationQuinone preparationHypericinSynthesis methods
The invention relates to a method for synthesizing hypericin. In the method, emodin serves as a raw material; emodin anthrone condensation reaction with low yield is optimized by a microwave-assisted synthesis method under the alkaline condition; the microwave heating temperature is 130 to 180 DEG C; and the reaction time is 0.2 to 1 hour. Under the conditions, the yield of the condensation reaction is increased by multiple times compared with that of the conventional method; reaction time is greatly saved; the yield of the reaction at each step is over 80 percent; and the reaction conditionscan meet the kilogram-grade production scale of a targeted compound. The synthetic route and method has the characteristics of high yield, short reaction route, mild reaction conditions, short reaction time, low synthetic cost and the like.
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Novel quinones as disease therapies
Novel quinones are provided, as well as compositions comprising these novel quinones. Methods of using the novel quinones in treatment of various indications including cancer are also provided.
Owner:CELLGATE
Preparation method of high optical purity shikonin and alkannin, and derivatives thereof
InactiveCN102399139AHigh optical purityHigh yieldOrganic compound preparationCarboxylic acid esters preparationSide chainEnantiomer
The invention discloses a preparation method of high optical purity shikonin and alkannin, and derivatives thereof. According to the invention, an intermediate separation means is used to prepare the high optical purity shikonin and alkannin, and the derivatives thereof, wherein the intermediate separation is that an amide diastereomer is formed from a carboxyl-contained intermediate and chiral amine, and separation is carried out through column chromatography or a recrystallization process. The derivatives of shikonin and alkannin are tetramethoxylated derivatives with shikonin and alkannin as mother nuclei, dimethoxylated-2-sidechainisomer derivatives and dimethoxylated-6-sidechainisomer derivatives with shikonin and alkannin as mother nuclei, 1,4-diacetoxylated-5,8-dimethoxylated-2-sidechainisomer derivatives and 1,4-diacetoxylated-5,8-dimethoxylated-6-sidechainisomer derivatives with shikonin and alkannin as mother nuclei, and diacetoxylated-6-sidechainisomer derivatives and diacetoxylated-2-sidechainisomer derivatives with shikonin and alkannin as mother nuclei. The preparation method of the invention, which has the advantages of easily available raw material, low price and high yield of each step reaction, is suitable for the large scale preparation.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing 2-ethylanthraquinone by continuous 2-(4-alkylbenzoyl)benzoic acid ring-closing reaction
InactiveCN103360229AEasy to operateReduce labor intensityOrganic compound preparationQuinone preparationBenzoic acidAnthraquinones
The invention discloses a method for preparing 2-ethylanthraquinone by continuous 2-(4-alkylbenzoyl)benzoic acid ring-closing reaction, which comprises the following steps: mixing liquid 2-(4-alkylbenzoyl)benzoic acid and room-temperature concentrated sulfuric acid in a mixer in a mass-volume ratio of 1:1.5-1:2.5, wherein the mixing temperature is room temperature to 100 DEG C, and the retention time of the mixture in the mixer is 1-10 minutes; connecting the mixer with a stirring reactor, and carrying out ring-closing reaction, wherein the temperature of the stirring reactor is 100-140 DEG C, and the retention time of the reaction materials in the reactor is 5-30 minutes; and directly hydrolyzing the liquid after reaction, extracting, and distilling to obtain the 2-ethylanthraquinone. The detailed method is disclosed in the specification. The process is simple to operate and easy to control, lowers the labor intensity, improves the working environment, reduces the equipment investment, lowers the waste acid quantity, and enhances the product yield by 5-10% as compared with the existing production technique; and the product quality is good.
Owner:BEIJING INSTITUTE OF PETROCHEMICAL TECHNOLOGY +1
Material for organic electroluminescent device and organic electroluminescent device using the same
InactiveCN101410364ASolution to short lifeOrganic compound preparationElectroluminescent light sourcesQuinoneAryl
A material for an organic electroluminescent device including a quinone derivative represented by the following formula (1), (2) or (3): wherein R<1 >to R<16 >are each a hydrogen atom, a halogen atom, a cyano group, an alkoxy group, a substituted or unsubstituted aryloxy group, an alkyl group, a fluoroalkyl group, an aryl group or a heterocyclic group; provided that at least one of R<1 >to R<4>, at least one of R<5 >to R<10 >or at least one of R<11 >to R<16 >is an aryloxy group; and X is a substituent represented by any one of the following formulas (a) to (f): wherein R<17 >to R<19 >are a hydrogen atom, an alkyl group, or aryl group; and R<18 >and R<19 >may be bonded together to form a ring.
Owner:IDEMITSU KOSAN CO LTD
A kind of PD/C catalyst and preparation method for TMBQ hydrogenation production TMHQ
InactiveCN102294240AEasy to makeHigh catalytic activityOrganic compound preparationQuinone preparationWater bathsActivated carbon
The invention relates to a Pd / C catalyst for producing 2,3,5-trimethylhydroquinone (TMHQ) by virtue of hydrogenation of 2,3,5-trimethylbenzoquinone (TMBQ) and a preparation method thereof. In the Pd / C catalyst provided by the invention, a noble metal Pd supported on a carrier activated carbon exists in a nano particle form, the dispersion degree of Pd is not less than 30%, and the carrier activated carbon has micropores and mesopores. The preparation method comprises the following steps: (1) carrying out acid treatment on the carrier activated carbon, and adding an acid solution in activated carbon for carrying out water bath reflux treatment; (2) washing activated carbon treated by the acid with deionized water to be neutral, and drying so as to obtain the activated carbon carrier; (3) infiltrating the activated carbon carrier with an infiltration liquid in advance; (4) slowly dropwise adding a 0.01-0.3mol / L Pd source solution in the activated carbon carrier which is infiltrated in advance, so that Pd is supported on the activated carbon, thus a catalyst precursor is obtained; and (5) drying the catalyst precursor and then treating the dried catalyst precursor by a reduction method so as to obtain the Pd / C catalyst for producing TMHQ by virtue of hydrogenation of TMBQ. The Pd / C catalyst provided by the invention has the characteristics of being simple and efficient, and having high catalysis property in BTOP (benzene to phenol) reaction.
Owner:ZHEJIANG NORMAL UNIVERSITY
Oxygen alkylated derivative of parent nucleus of alkannin naphthazarin and preparation method and application thereof
InactiveCN101781308AInhibition of tumor growthCytotoxicity decreased or disappearedOrganic compound preparationCarboxylic acid esters preparationAlkaneOxygen
The invention relates to an oxygen alkylated derivative of a parent nucleus of alkannin naphthazarin and a preparation method and application thereof. The structural formula of the oxygen alkylated derivative of the parent nucleus of alkannin naphthazarin is particularly shown as in the following formula, wherein R is H, alkane with 1-10 carbon atom(s), olefin, acrne or COR', R' is H, alkane with 1-10 carbon atom(s), olefin or acrne. The invention also relates to the application of the oxygen alkylated derivative of the parent nucleus of alkannin naphthazarin in preparation of anticancer drugs. The preparation method of the invention has the advantages of easily accessible raw materials, short synthetic route and high reaction yield, and the prepared compound can be used as a prodrug for treatment of malignant tumors.
Owner:SHANGHAI JIAO TONG UNIV
General method for functionalizing carbon nanotubes via solvent free diels-alder reactions
InactiveUS20160152477A1Conductivity recoveryImprove conductivityCarboxylic acid nitrile preparationConductive materialChemical reactionGas phase
The present invention provides methods by which carbon nanotubes can be functionalized via Diels-Alder reactions under solvent free conditions. Such methods include reacting carbon nanotubes with Diels-Alder dienes or dienophiles to obtain adducts that includes the diene or dienophile moiety bound to the carbon nanotubes. Functionalized carbon nanotubes and dispersions containing functionalized carbon nanotubes are provided. The present invention provides functionalization methods of carbon nanotubes through gas phase, liquid phase, or solid phase reactions without any solvents other than the reactants. Such processes are also amenable to a wide variety of chemical reactions that use other functionalizing agents. Additionally, such methods are cost effective, easily scalable and can provide for functionalized CNTs in large, industrial-scale quantities.
Owner:SHANDONG DAZHAN NANO MATERIALS
Preparation method of spherical micron-size gamma-aluminium oxide carrier for preparing hydrogen peroxide by anthraquinone hydrogenation
InactiveCN108325537ALarge specific surface areaMany active sitesOrganic compound preparationQuinone preparationAir atmosphereCalcination
The invention relates to a preparation method of spherical micron-size gamma-aluminium oxide carrier for preparing hydrogen peroxide by anthraquinone hydrogenation. The preparation method comprises the following steps: dispersing anhydrous glucose and Al(NO3)3.9H2O into deionized water, carrying out ultrasonic treatment for 10min, adding a urea water solution, heating the mixed solution at 180 DEGC for 8 hours, alternately washing the hydrothermal product with deionized water and ethanol until the filtrate is clear; drying the filter cake in a blower drying oven at 80 DEG C for 8 hours to obtain a colloid carbon / AlOOH complex, and carrying out calcination in an air atmosphere of 550 DEG C for 3 hours to obtain spherical micron-size gamma-Al2O3 powder, taking the powder as a carrier, supporting an active component Pd- by adopting a incipient wetness method, then carrying out washing, drying and calcination to obtain a Pd / gamma-Al2O3 catalyst, wherein before the Pd- is supported, auxiliary agents such as Ni-, Fe- and Zn- can also be supported by adopting the same incipient wetness method of the Pd-. The Pd / gamma-Al2O3 catalyst exhibits high hydrogenation efficiency in an anthraquinone hydrogenation reaction, and the hydrogenation efficiency can reach 11.30g H2O2 per liter of a working fluid.
Owner:WUHAN UNIV OF TECH
Method for preparing 2-alkyl anthraquinone by taking solid super acids as catalysts
InactiveCN108299176AHigh catalytic efficiencyReduce usageOrganic compound preparationPreparation from carboxylic acid anhydridesBenzoic acidHeteropoly acid
The invention provides a method for preparing 2-alkyl anthraquinone by taking solid super acids as catalysts. The 2-alkyl anthraquinone (alkyl is straight or branched alkyl with the number of carbon atoms of 1-6) is obtained by adopting the solid super acids like perfluorinated sulfonic acid resin and heteropoly acid as the catalysts, taking 2-(4'-alkyl benzoyl) benzoic acid as a raw material andperforming acylated dewatering closed loop through Friedel-Crafts reaction. The method provided by the invention is characterized in that traditional smoking sulfuric acid catalysts are replaced by the solid super acids, so that the environment is friendly, and no waste acid is discharged; the operation process is simple, and the solid catalysts are easy to recover; therefore, the method is a green pollution-free new process.
Owner:BEIJING UNIV OF CHEM TECH
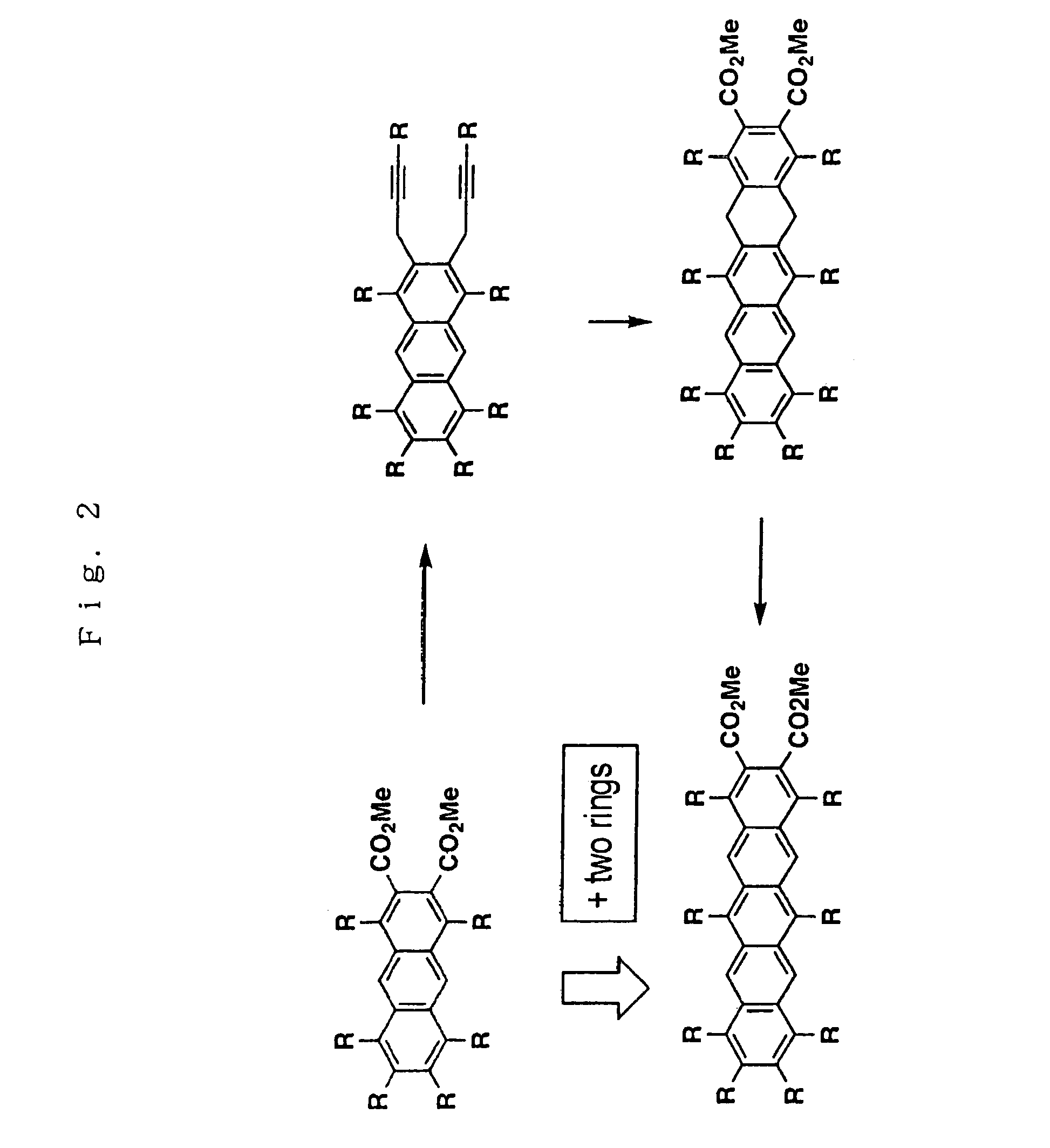
Polyacene derivatives and production thereof
InactiveUS7901594B2Improve solubilityEasy to implementConductive materialSolid-state devicesBenzeneHalogen
The present invention relates to polyacene derivatives represented by general formula (I) below:(wherein R1 to R10, etc. each represents hydrogen atom, hydrocarbon group, or an alkoxy group; A1 and A2 are hydrogen atom, a halogen atom, a hydrocarbon group, an alkoxy group, cyano group, etc.; n is an integer of not less than 1; R6 and R7 may be linked to each other to form a ring); and a process for preparing the polyacene derivatives from polyhydro compounds as well as electrically conductive materials comprising the polyacene derivatives. According to the process for preparing the polyacene derivatives of the present invention, optional substituents can be introduced into any carbon atoms of the polyacene, and the number of aromatic rings can be increased.
Owner:JAPAN SCI & TECH CORP
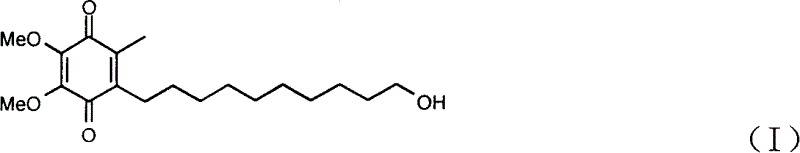
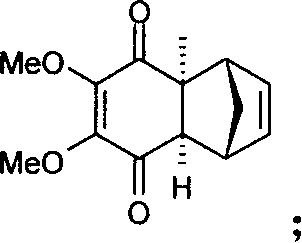
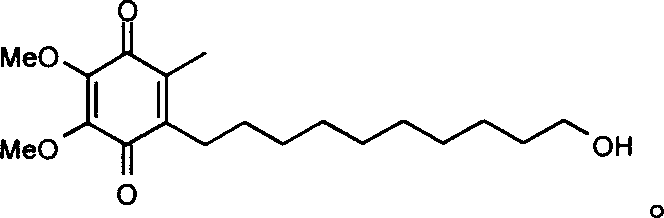
Method for synthesizing Idebenone
InactiveCN1696096AEasy to operateNot dangerousOrganic compound preparationQuinone preparationIdebenoneRetro-Diels–Alder reaction
Owner:SHANGHAI RECORD PHARM CO LTD +1
Popular searches
Lamp details Semiconductor/solid-state device manufacturing Natural mineral layered products Halogenated hydrocarbon preparation Luminescent compositions Organic semiconductor devices Group 3/13 element organic compounds Organic conductors Phosphorus organic compounds Analysis using nuclear magnetic resonance
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com