Preparation method of high optical purity shikonin and alkannin, and derivatives thereof
A technology of optical purity and shikonin, applied in the field of medicine and chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Synthesis of ethyl 3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene)propionate (2)
[0061] Under the protection of nitrogen, 1 mol of ethyl bromoacetate was dropped into the tetrahydrofuran solution of activated zinc powder (2 mol). -2-Naphthaldehyde tetrahydrofuran solution, after addition, continue to reflux for 2 hours, cool, add 200ml of water, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 0.92mol 3-hydroxyl-3 -(1,4,5,8-Tetramethoxy-2-naphthalene) ethyl propionate, yield 92%. 1 H-NMR (300MHz, CDCl 3 ): d 1.27(t, J=8.1, 3H, -CH2 CO 2 CH 2 C H 3 ), 2.7-2.9(m, 2H, -C H 2 CO 2 CH 2 CH 3 ), 3.78(s, 3H, ArOC H 3 ), 3.90(s, 3H, ArOC H 3 ), 3.94(s, 3H, ArOC H 3 ), 3.96(s, 3H, ArOC H 3 ), 4.20(dd, J 1 =6.9,J 2 =13.8, 2H, -CH 2 CO 2 C H 2 CH 3 ), 5.61(m, 1H, -C H OHCH 2 -), 6.82(s, 2H, Ar H ), 7.08(s, 1H, Ar H ).
Embodiment 2
[0062] Example 2: Synthesis of 3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene)propionic acid (3)
[0063] Dissolve 0.5mol 3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene) ethyl propionate in methanol, add 100ml of 6M sodium hydroxide, reflux for 1h, add 6M Hydrochloric acid 100ml, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain an equivalent amount of 3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene) Propionic acid was directly used in the next reaction without further purification. 1 H-NMR (300MHz, CDCl 3 ): d 2.90(m, 2H, -C H 2 CO 2 H), 3.76(s, 3H, ArOC H 3 ), 3.90(s, 9H, 3×ArOC H 3 ), 5.65(m, 1H, -C H OHCH 2 -), 6.81(s, 2H, Ar H ), 7.04(s, 1H, Ar H ).
Embodiment 3
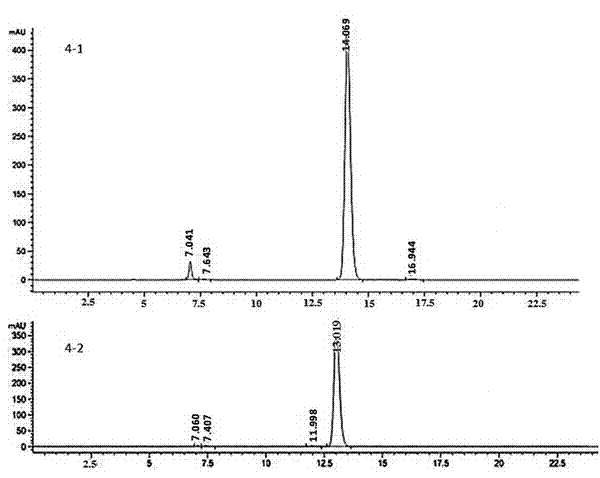
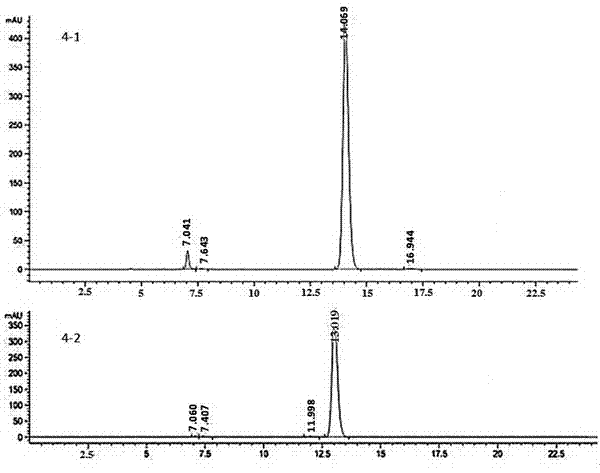
[0064] Example 3: (R, S)-3-hydroxyl-3-(1,4,5,8-tetramethoxy-2-naphthalene)-N-(1-phenylethyl)-propionamide (4- 1) and (S,S)-3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene)-N-(1-phenylethyl)-propionamide (4-2 )Synthesis
[0065] 0.5mol 3-hydroxy-3-(1,4,5,8-tetramethoxy-2-naphthalene)propionic acid, 0.5mol (S)-α-methylbenzylamine, 0.55mol benzotriazole -1-yloxytris(dimethylamino)phosphonium hexafluorophosphate and 1.1mol triethylamine were dissolved in 100ml of anhydrous N,N-dimethylformamide, stirred at room temperature for 2h, and N, N-dimethylformamide was added with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was subjected to column chromatography to obtain 0.40 mol (R, S)-3-(tert-butyl Dimethylsilyloxy)-3-(1,4,5,8-tetramethoxy-2-naphthalene)-N-(1-phenylethyl)-propionamide (4-1), yield 40 %. [α] 20 D =1.975; 1 H-NMR (300MHz, CDCl 3 ): d 1.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com