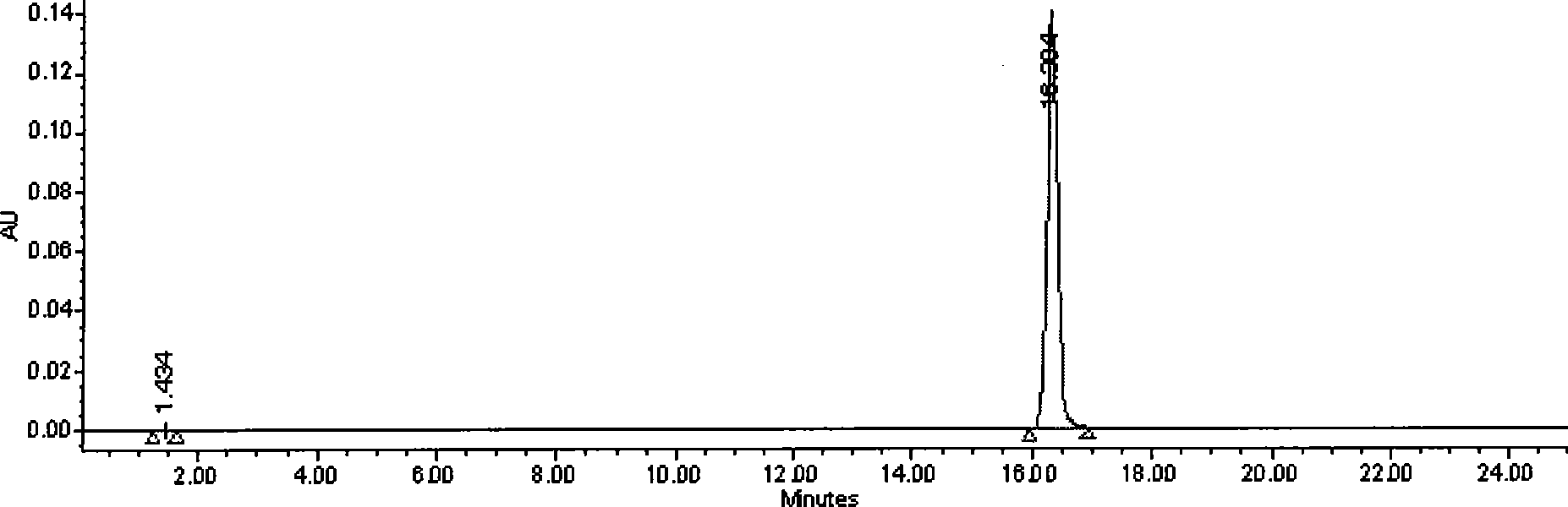
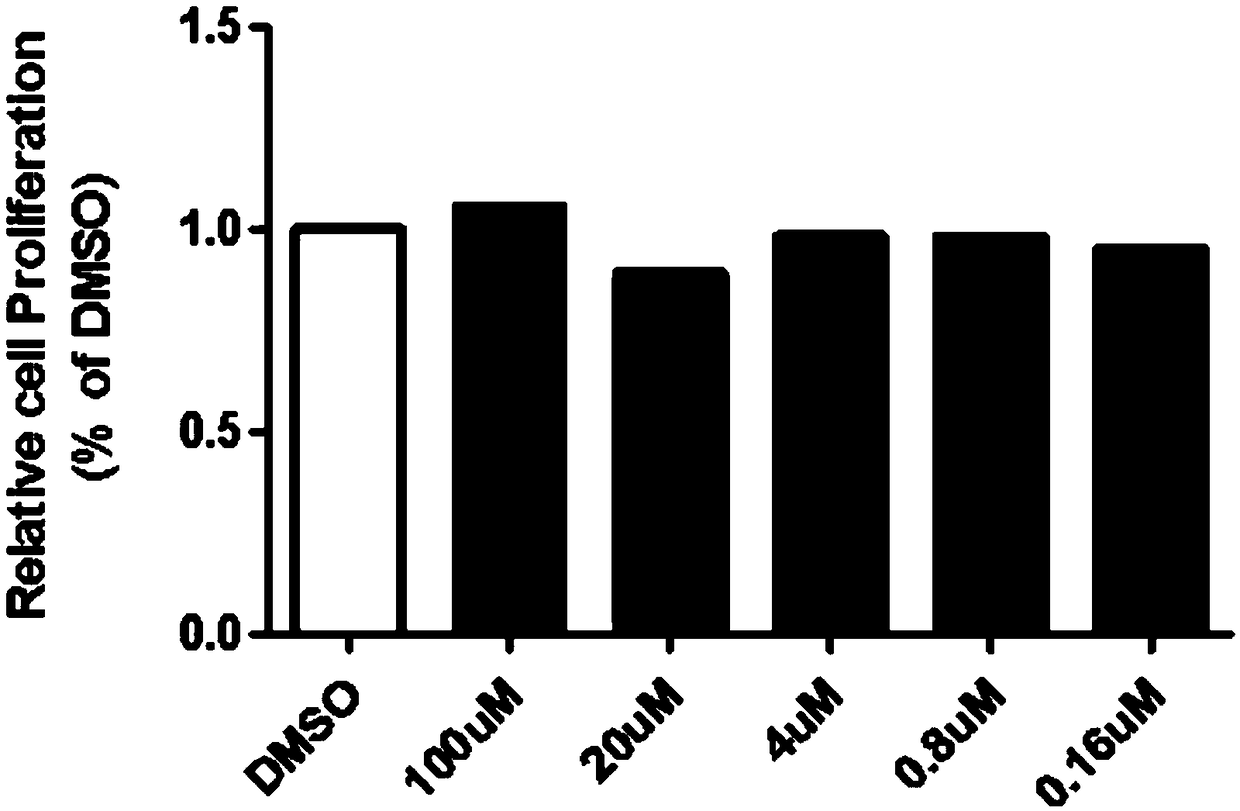
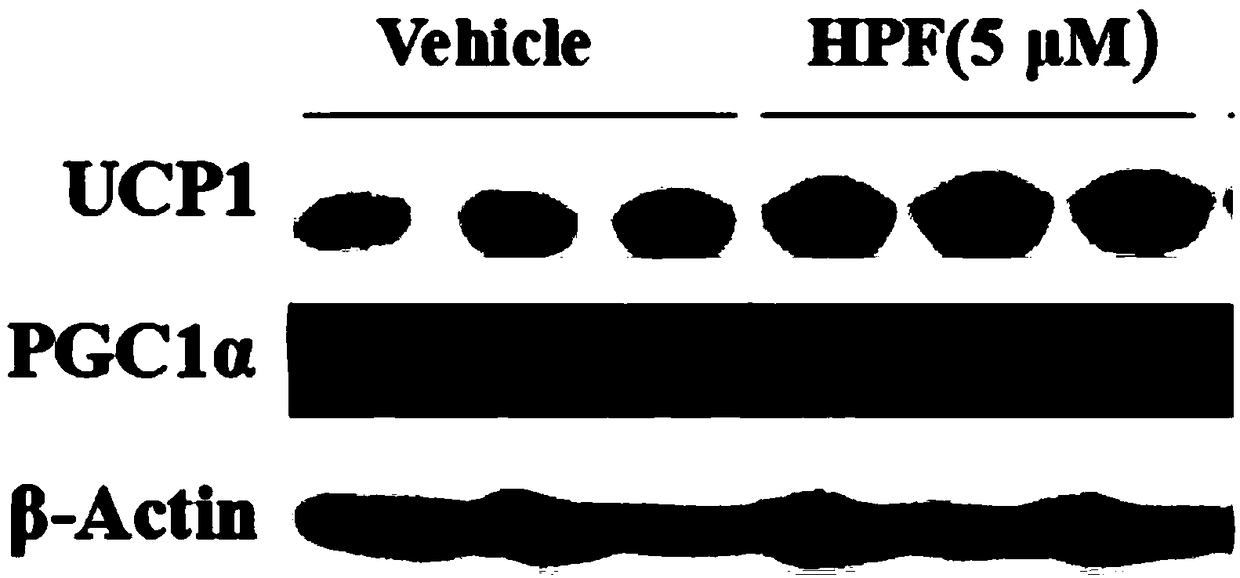
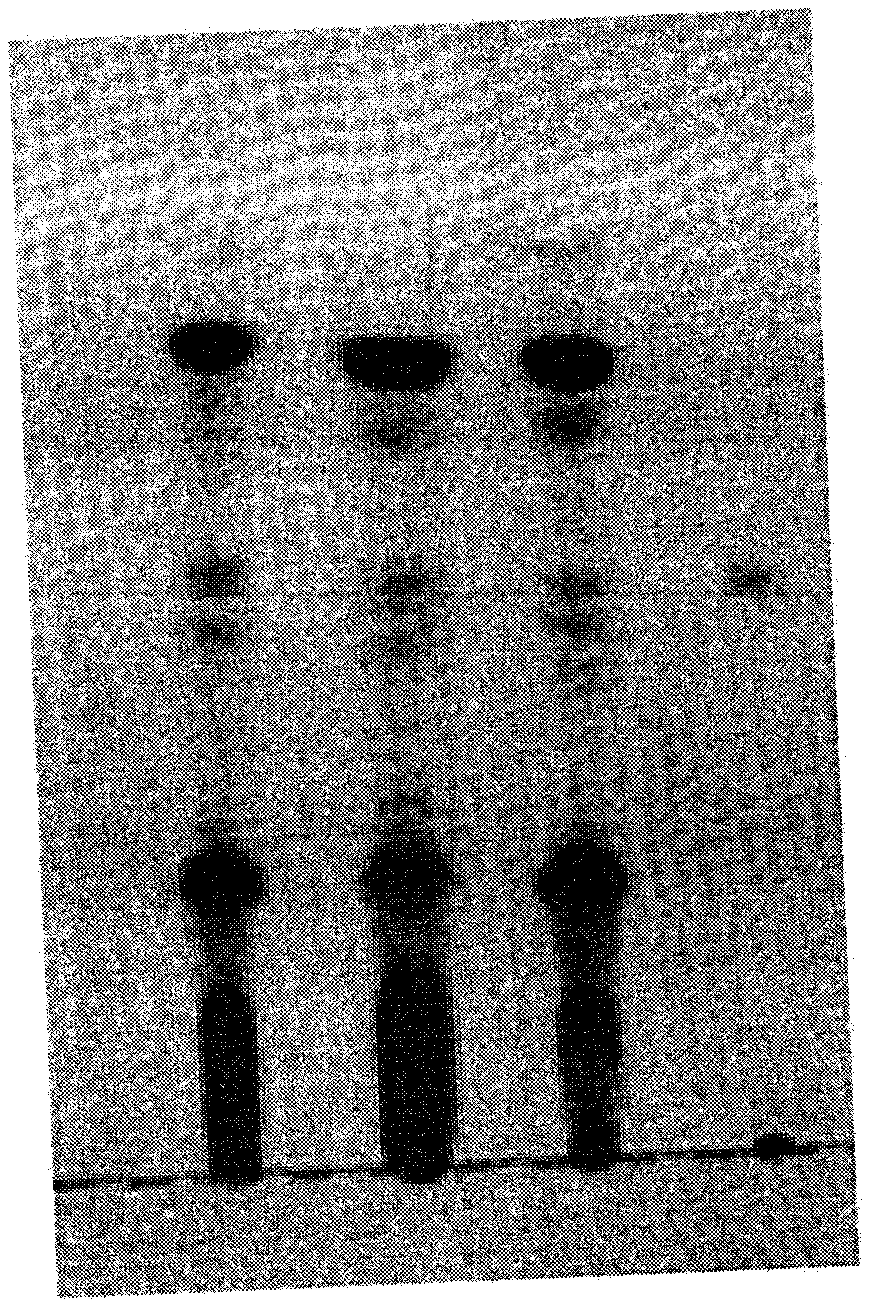
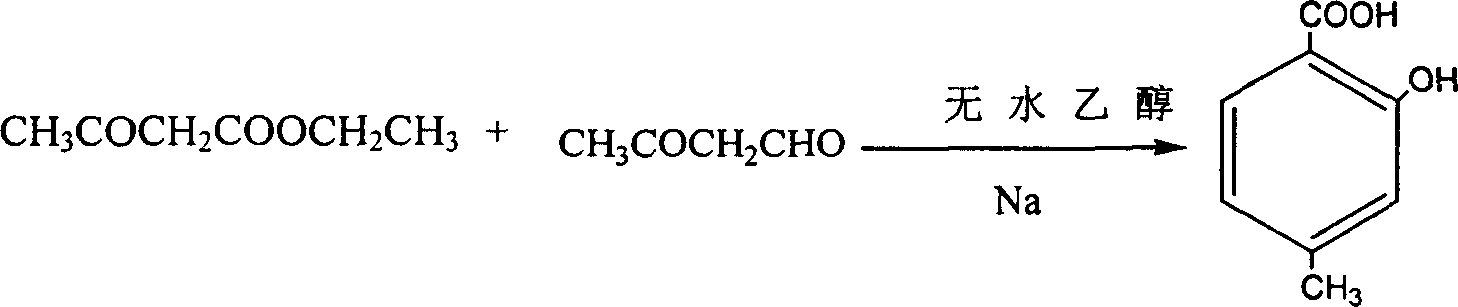Patents
Literature
190 results about "Hypericin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
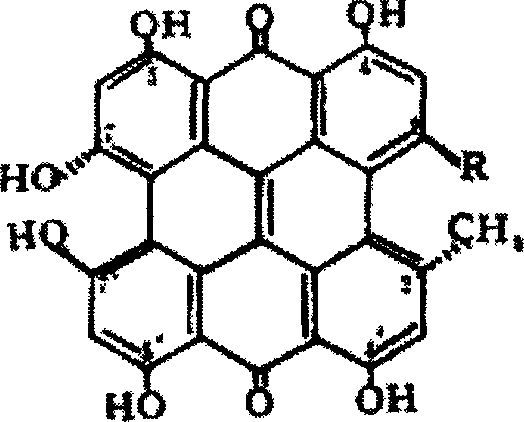
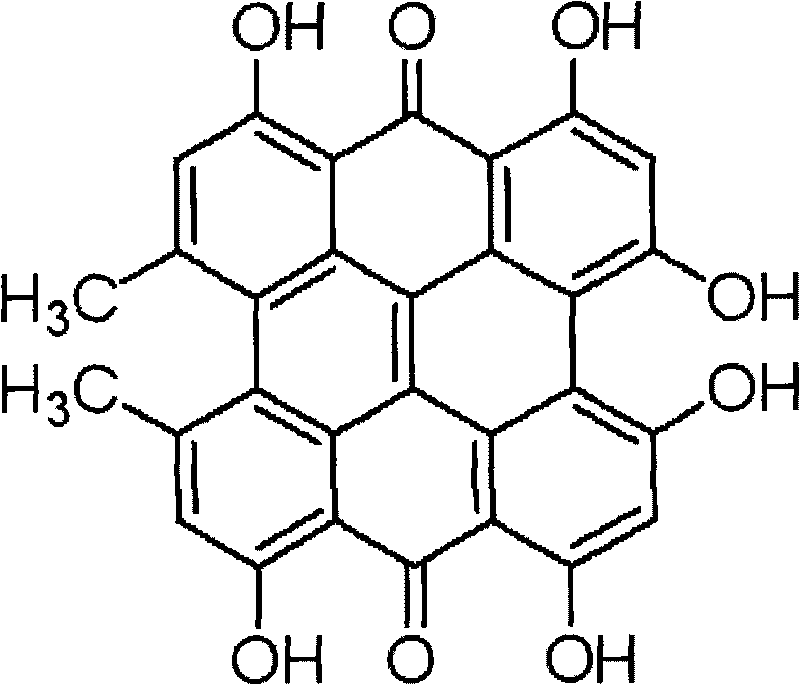
Hypericin is a naphthodianthrone, an anthraquinone derivative which, together with hyperforin, is one of the principal active constituents of Hypericum (Saint John's wort). Hypericin is believed to act as an antibiotic, antiviral and non-specific kinase inhibitor. Hypericin may inhibit the action of the enzyme dopamine β-hydroxylase, leading to increased dopamine levels, although thus possibly decreasing norepinephrine and epinephrine.
Preparation method of hyperin and hypericin of Hypericum perforatum
InactiveCN1880328AHigh yieldReduce manufacturing costOrganic active ingredientsSenses disorderHypericum perforatumHyperoside
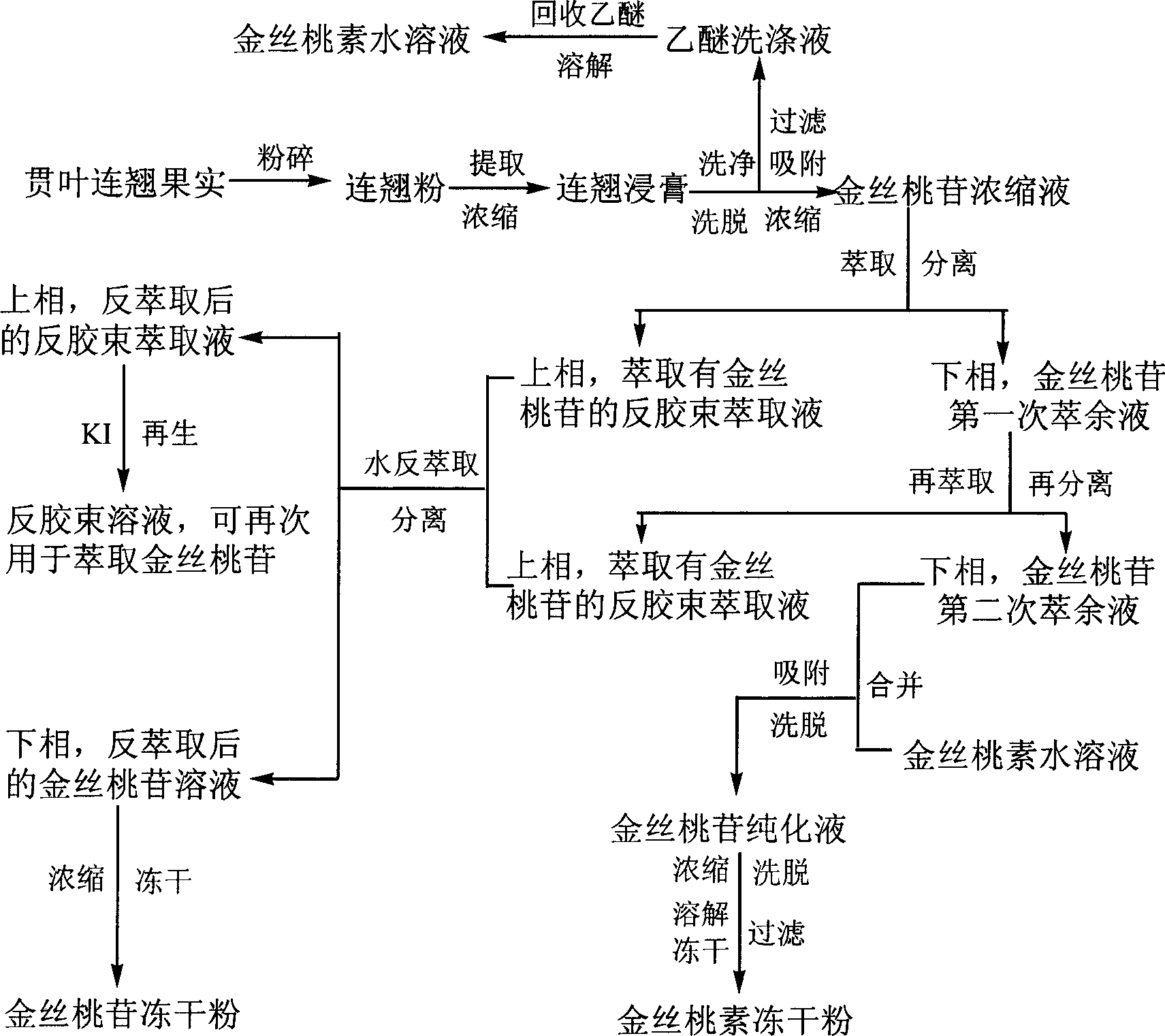
This invention relates to a method for producing Hypericum perforatum hyperoside and hypericin. This invention comprehensively utilizes Hypericum perforatum fruits which are rich in our country to produce both hyperoside and hypericin pharmaceutical active components with high recovery rate, high product purity, and low cost. During the producing process, it achieves the recovery and cycling use of many solvents like ethanol and ethyl ether, and also achieves the regeneration and cycling use of micelle reextracting agent and absorption resin. The plant biological active compounds produced by this invention can be broadly used in heat-clearing and detoxifying, astringing to arrest bleeding and promote diuresis, and antidepressant drug.
Owner:CHONGQING UNIV
Biological preparation method of Hypericum perforatum L extractive
InactiveCN101829164AIncrease profitIncrease hypericin contentNervous disorderAntiviralsWater bathsActive enzyme
The invention relates to a biological preparation method of a Hypericum perforatum L extractive, providing a preparation method which can utilize medicinal material resources adequately, has simple process, is suitable for large-scale production and can prepare the Hypericum perforatum L extractive in accordance with or higher than international standards. The method comprises the following steps of: drying the Hypericum perforatum L firstly and smashing into Hypericum perforatum L powder, then weighing enzyme, adding warm water in the enzyme to dissolve the enzyme, activating enzyme solution by water bath, mixing the Hypericum perforatum L powder with water, performing water bath, soaking, cooling, adjusting pH with hydrochloric acid, stirirng uniformly, heating by water bath, adding the actived enzyme solution, mixing uniformly, performing enzymolysis, filtering, abandoning the filter to obtain medicinal material powder after enzymolysis, adding ethanol and fluxing for extraction, filtering, combining the filtrate, condensing under reduced pressure, recycling ethanol, and drying to obtain the Hypericum perforatum L extractive. The invention improves the content of hypericin in the extractive and the abandoned filtrate after enzymolysis takes away a large amount of water-soluble impurities so that the moisture of the extractive is not needed to absorb again. The biological method is safe, pollution free and has simple operation and is easy for industrialized application.
Owner:HENAN UNIV OF CHINESE MEDICINE
Photosensitive biological germicide of perylene quinone and its prepn.
The present invention relates to a perylene quinone derivative light-sensitive biological fungicide and its preparation method. It is characterized by that its contained main component is organic solvent extract solution of a series of derivative which uses 4-hydroxy-3,10-perylene quinone as active centre and includes hypocrellin A.B. elsinochrome A.B.C, phleichrome, cercosporin, cladosporin A,B,C,D, erythroaphin A,B, hypericin and stentorin, etc. and its auxiliary component is non-ionic surfactant, and the solvent is water. Its weight composition is main component; auxiliary component=1:1-3, the rest is water. It can effectively inhibit the growth of various fungi, and is applicable to various vegetables, fruits and flowers.
Owner:张红雨 +1
Chinese medicinal herb preparation for poultry and application thereof
ActiveCN102600383AImprove non-specific immunityReduced feed intakeOrganic active ingredientsImmunological disordersFecesHypericum perforatum
The invention discloses a Chinese medicinal herb preparation for poultry. The effective constituents of a Chinese medicinal herb product disclosed by the invention are made up of echinacea purpurea, honeysuckle, hypericum perforatum, glycyrrhiza, folium isatidis, platycodon grandiflorum, lophatherum gracile, mint and hypericin. The Chinese medicinal herb preparation disclosed by the invention can relieve the exterior syndrome with drugs pungent in flavor and cool in property and clear away heat and toxic materials; and the main therapeutical effects include strengthening vital Qi and eliminating pathogens, balancing Yin and Yang, and improving non-specific immunity of organisms. The Chinese medicinal herb preparation for poultry has significant therapeutical effect for depressed spirit of poultry, feed intake decrease, egg laying reduction, eyelid swelling of poultry caused by unknown viruses, respiratory inflammation, yellow-green dung, weight increment decline, soft-shelled eggs, and death rate increment, etc.; furthermore, the Chinese medicinal herb preparation for poultry can be utilized to prevent the diseases of untypical Newcastle disease and avian influenza etc. and treat the relevant mixed infection, secondary infection, untypical diseases and chronic cases; and finally, the Chinese medicinal herb preparation for poultry well promotes the recovery of production performance after the poultry is healed.
Owner:HEILONGJIANG HUIFENG ANIMAL HEALTH PRODS +1
Method for synthesizing hypericin
ActiveCN102180782AHigh yieldShort reaction pathOrganic compound preparationQuinone preparationHypericinSynthesis methods
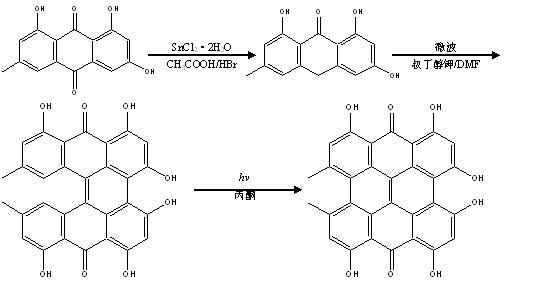
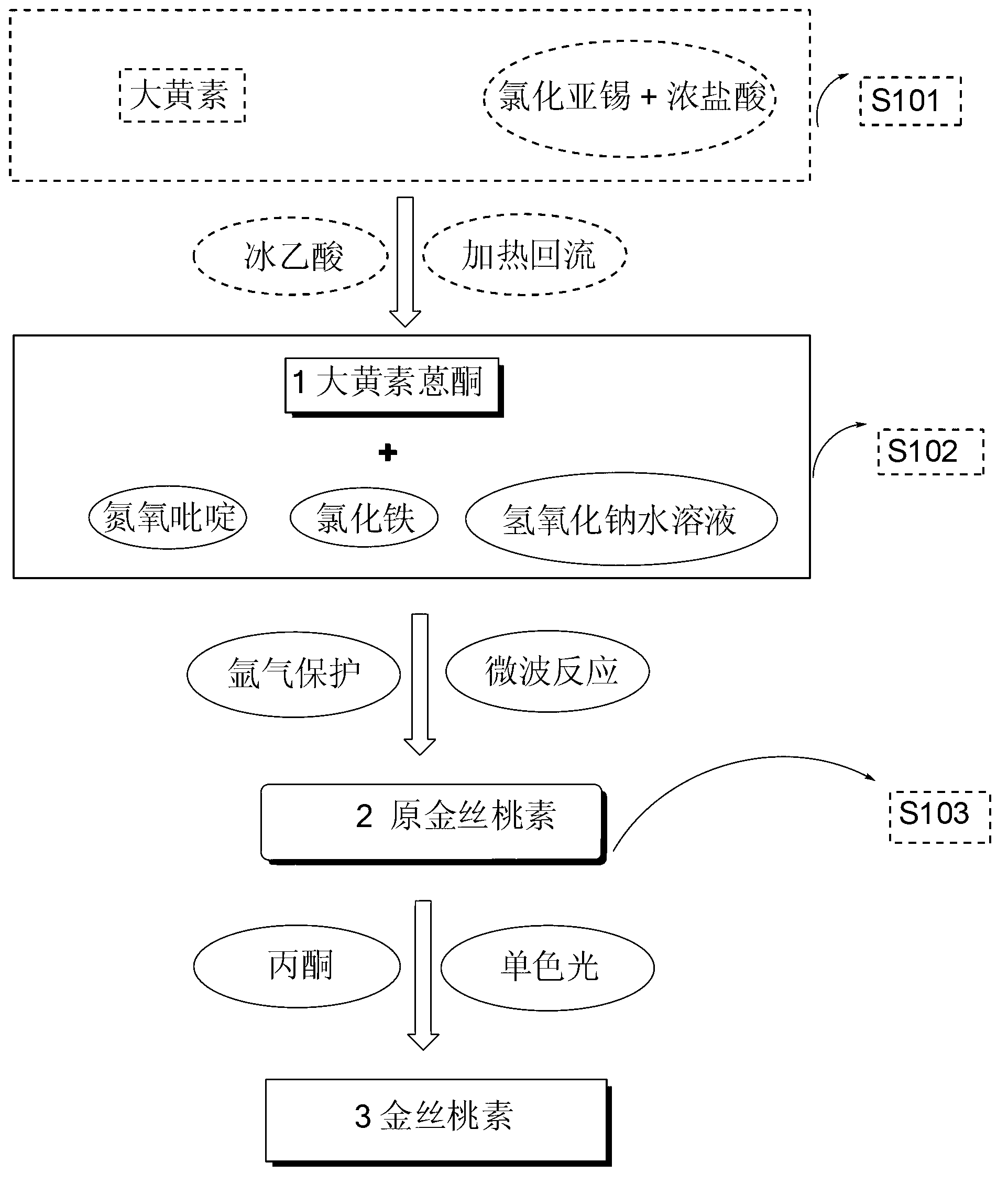
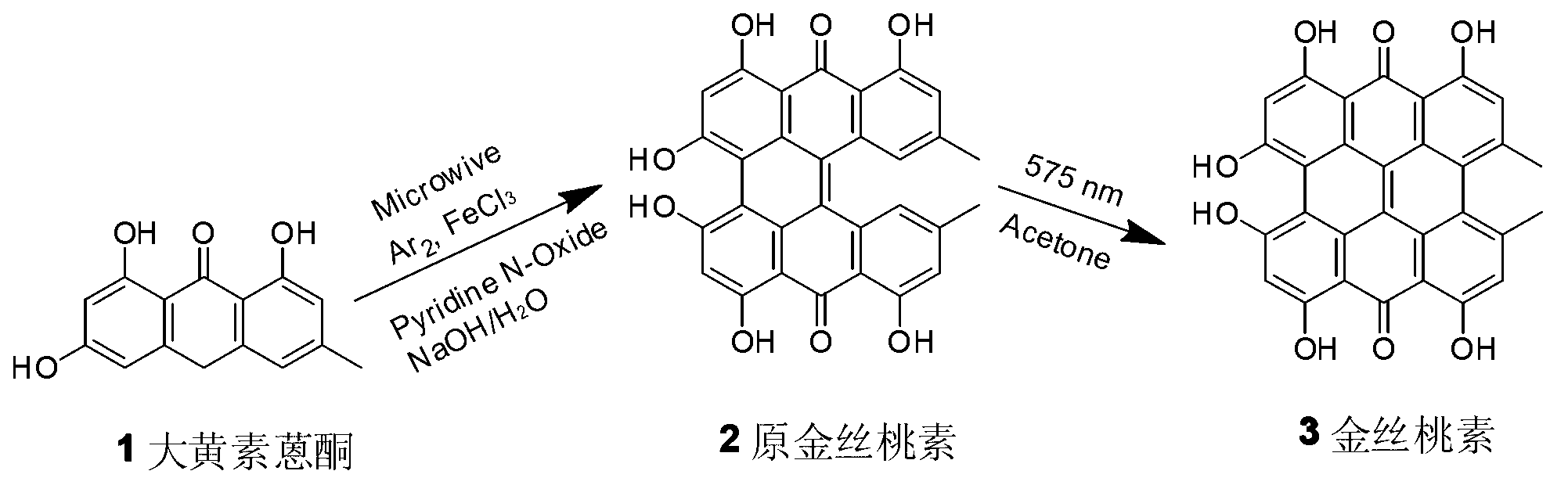
The invention relates to a method for synthesizing hypericin. In the method, emodin serves as a raw material; emodin anthrone condensation reaction with low yield is optimized by a microwave-assisted synthesis method under the alkaline condition; the microwave heating temperature is 130 to 180 DEG C; and the reaction time is 0.2 to 1 hour. Under the conditions, the yield of the condensation reaction is increased by multiple times compared with that of the conventional method; reaction time is greatly saved; the yield of the reaction at each step is over 80 percent; and the reaction conditionscan meet the kilogram-grade production scale of a targeted compound. The synthetic route and method has the characteristics of high yield, short reaction route, mild reaction conditions, short reaction time, low synthetic cost and the like.
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Process for preparing hypericum perforatum extract
InactiveCN1392129AGood antidepressant activityIncrease polarityQuinone separation/purificationUnknown materialsHypericinActive component
The present invention relates to the preparation process of extract containing three active components including hypericin, Hyperforin and flavones in the total content not less than 50% from hypericum perforatum or other congeneric plant. The three active components are separated from the polar solvent extracted liquid via biphase extraction with non-polar solvent selected based on their difference in polarity. They are then purified separately based on their difference in acid-base property to obtain the extract A with high hypericin and flavones content and extract B with high Hyperforin content. The two extracts are merged through dissolution to obtain hypericum perforatum extract with total active component content not less than 50%. The extract has the obvious activity of resisting depression and may be used as the material of depression treating medicine or functional food.
Owner:SUN YAT SEN UNIV
Chemical synthesis process for hypericin and derivatives thereof
InactiveCN1827574AMeet needsSimple and fast operationOrganic compound preparationCarbonyl compound preparationChemical synthesisOxygen
The invention discloses a method for chemosynthesis of the hypericin and its derivant. The said method includes the following steps: 1) dissolving 1, 3, 8-trihydroxy-6-methyl anthraquinone in the analytical pure glacial acetic acid,then adding concentrated hydrochloric acid with 36-40 % of zinc bichloride dehydrate, reacting for 1-4 hours at the temperature of less than 125 DEG C, cooling, and obtaining the 1, 3, 8-trihydroxy-6-methyl anthranone; 2) making 1, 3, 8-trihydroxy-6-methyl anthranone, pyridine, piperidine, nitrogen-oxygen pyridine and ferrisulphas hepthydrate react free of light for 0.5-3 hours at the temperature of 100-130 DEG C, then dissolving the reaction products in the acetone, illuminating them by a halogen lamp for 12-24 hours, thickening and dissolving them in the skellysolve B, filtering the deposition, and obtaining the hypericin. The method has advantages of that its operation is simple and reasonable, its cost is cheap, its productivity is high, its actual applied value is higher, so its actual value is broad.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method for extracting hypericin from Hypericum perforatum and medicine made up using hypericin as main composition
The method for extracting hypericin from hypericum perforatum includes the following steps: soaking plant hypericum perforatun in KOH and NaHCO3 solution with a specific concentration, boiling, filtering and drying to obtain its extract, dissolving said extract in propyl alcohol, filtering solute, and using water-alcohol process to refine the extract so as to obtain the invented product. It is used as main component, and matched with other component, and can be made into the hypericin medicine composite or other different dosage forms for curing depression.
Owner:牛勃
Method for preparing hypericins
InactiveCN101759549AFully extractedFully liquefiedQuinone separation/purificationHypericinHypericum perforatum
The invention relates to a method for separating and purifying hypericins by adopting a macroporous resin separation technique and an alumina chromatographic technique. The method comprises the following steps of: grinding fresh hypericum perforatum L, leaching the ground hypericum perforatum L for 1 to 2 hours in saturated lime solution, adjusting a pH value to 6 to 7 by hydrochloric acid at normal temperature, and performing resin separation, segmented elution, alumina chromatography, ethanol elution and cyclohexane to obtain the finished product. The method has the advantages of simple process, little energy consumption and high-purity hypericins.
Owner:NANJING ZELANG MEDICAL TECH
Extracting and purifying method for hypericum perforatum component in plant
InactiveCN1392130AIncrease contentReduce pollutionQuinone separation/purificationOrganic solventHypericin
The present invention relates to the process of extracting and purifying Hyperforin from Hypericum perforatum or other congenetic plant. The process includes poalr solvent extraction or biphase extraction or non-polar organic solvent extraction, impurity elimination with alcoholic water and acid and base treatment and the extract has Hyperforin content not less than 65%. Through further silica column chromatography and HPLC preparation, Hyperforin product with purity higher than 95% or 98% may be produced.
Owner:SUN YAT SEN UNIV
Method for extracting hypericin
InactiveCN1850766AIncrease contentLow toxicityNervous disorderQuinone separation/purificationHypericinOrganic solvent
This invention discloses hypericin distillation method. It includes following procedures 1. Leaf weeping forsythia is dried and pulverized. 2. The forsythia powder is mixed with organic solvent as volume ratio 1-2 t 3, then they are distilled at 40-70 degree centigrade for 2-4 hours, extracting solution is collected, filtrated and filtration liquor is collected. 3. The filtration liquor is decompressed and concentrated, concentrated solution is recovered. 4. The liquor is concentrated by resin, and then eluted, eluent is collected. 5. The eluent is decompressed and concentrated, and then hypericin extractive is got. In this invention its operation is simple, easy for industrial application; its distillation cost is low. The extracting solution toxity is little, distilling period is short. Hypericin content in extract is high. So this invention takes important part in hypericin organism distillation.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS +1
Preparation method for hypericin albumin nanometer particles
ActiveCN102525937AHigh effective contentSmall particle sizePowder deliveryOrganic active ingredientsHypericinFreeze-drying
The invention discloses a preparation method for hypericin albumin nanometer particles, which comprises (1) using bovine serum albumin as a carrier material to be dissolved in distilled water to manufacture carrier solution with the mass concentration of 10% to 40%; (2) dissolving hypericin in ethanol with the concentration more than 95% to produce oil phase with the mass concentration of 20% to 40%; (3) adding the oil phase having been fully stirred into the carrier solution, adjusting potential of hydrogen (PH) to 9, dropwise adding emulsifier to fully emulsify, then dropping glutaraldehyde to cure for more than 24 hours, and obtaining curing nanometer particle solution; (4) centrifuging at high speed of 15,000 rpm / min to 20,000 rpm / min, removing supernate, adding distilled water to perform ultrasonic dispersion, centrifuging again at high speed, removing supernate, washing with water and collecting, and drying in vacuum or freezing mode to achieve hypericin albumin nanometer particles. The hypericin albumin nanometer particles prepared with the method are good in stability on light and heat, and effective content of hypericin is improved.
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Method for extracting hypericin from Hypericum perforatum
InactiveCN105777522AReduce production processNo sticky lumpsQuinone separation/purificationBiotechnologyHypericin
The invention relates to a method for extracting hypericin from Hypericum perforatum. The method sequentially comprises the steps of enzyme treatment, ultrasonic assisted solvent extraction, and macro-porous adsorption resin separation and purification, and hypericin is efficiently extracted under optimized technological conditions, so the content of hypericin in obtained extract is improved. The extraction method has the advantages of easily available raw material, simple operation, low extraction cost, controllable production cost and wide industrial application prospect.
Owner:陕西笃诚医药科技有限公司
Process for extracting high-content hypericin from Hypericum perforatum L.
InactiveCN1951895ASimple processHigh in vitaminsQuinone separation/purificationHypericinHypericum perforatum
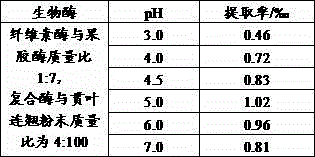
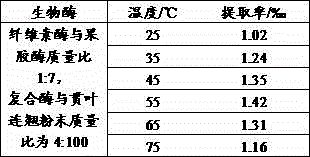
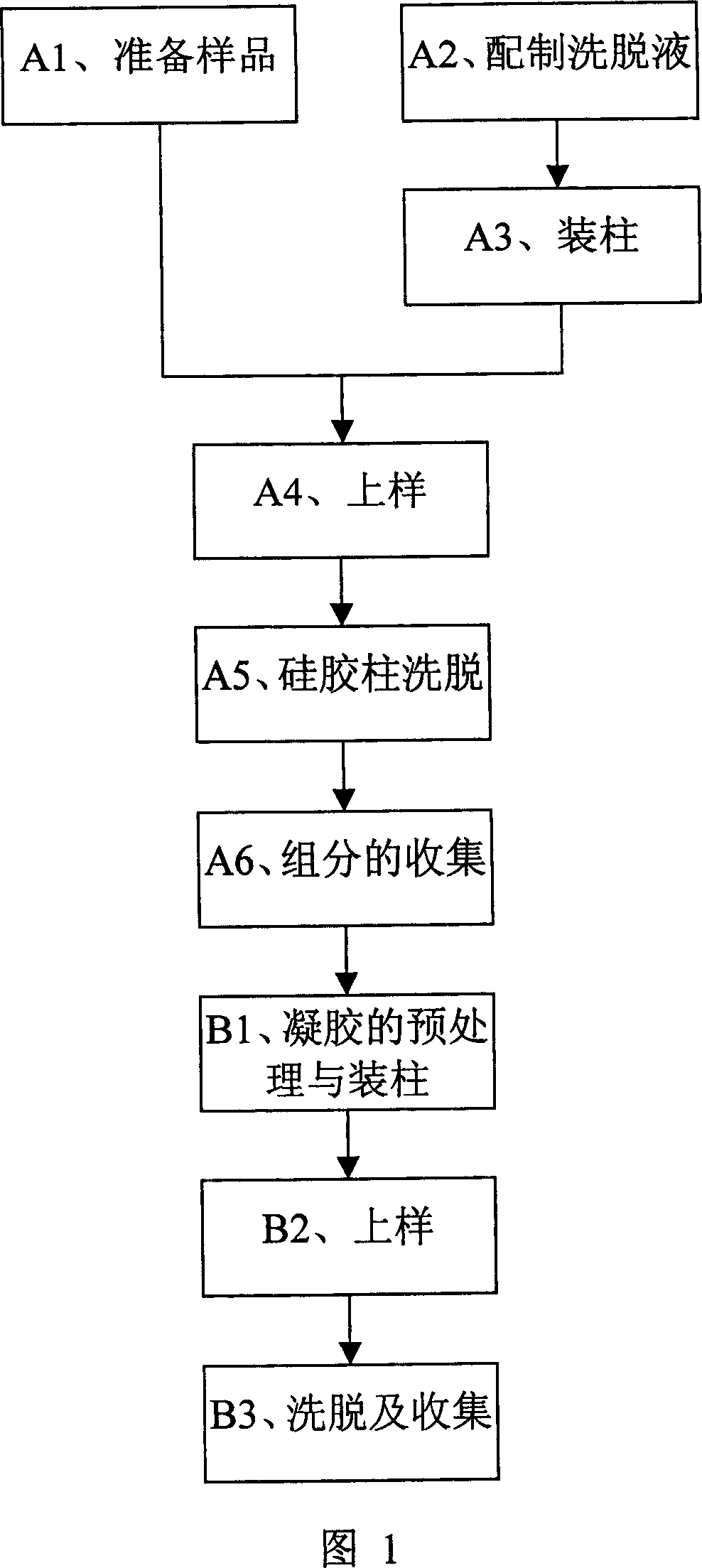
The invention discloses an extracting method of hypericin extract from Hypericum, which comprises the following steps: A1. Preparing sample; A2. Allocating eluent; A3. Loading column; A4. Sampling; A5. Eluting silica gel column; A6. Collecting group by group; B1. Predisposing gel; loading column; B2 sampling; B3. Eluting; collecting to obtain the product. The invention simplifies the technique with high extracting rate, which inhibits HepG2, A549, SpcA1 and MDA231 from growing effectively.
Owner:SHENZHEN POLYTECHNIC
Helianthrone derivatives as anti-cancer agents
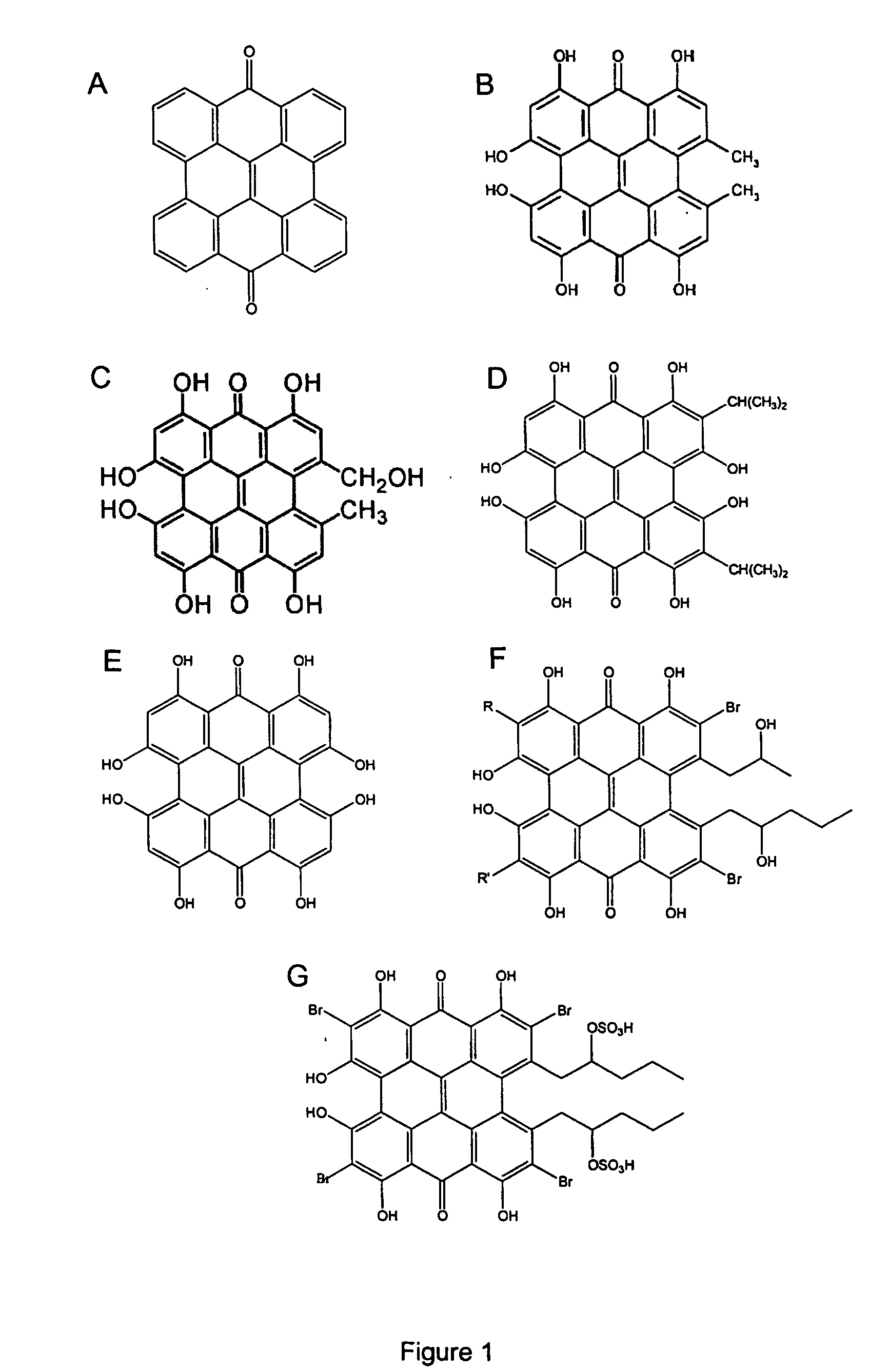
Hypericin, helianthrone and derivatives thereof of general formula (I) wherein the dotted line between positions 11 and 12 represent an optional C11-C12 bond; R is independently selected from the group consisting of hydroxy, C1-C10 alkoxy, NH—C1-C10 alkyl, and NH-hydroxy(C1-C10)alkyl; R′ is independently selected from the group consisting of hydroxy and C1-C10 alkoxy; R″ is independently selected from the group consisting of hydrogen, hydroxy, C1-C10 alkoxy, NH—C1-C10 alkyl, and NH-hydroxy(C1-C10)alkyl; and R1, R2, R3, R4, R5 and R6 are independently selected from the group consisting of hydrogen, hydroxy, chloro, bromo, C1-C10 alkyl, C1-C10 alkoxy, and C1-C10 alkoxycarbonyl, provided that R″ is not hydrogen when there is a C11-C12 bond, are useful as inhibitors of angiogenesis and can be used to prevent formation of metastases and restenosis and for the treatment of angiogenesis-associated ophthalmologic disorders. In addition, the helianthrones of formula (I) can be used for the treatment of tumors in the absence of light irradiation. New compounds include those of formula I which are other than hypericin and known hypericin derivatives and there is either a C11-C12 bond or at least one R″ is other than hydrogen.
Owner:NEW YORK UNIV +1
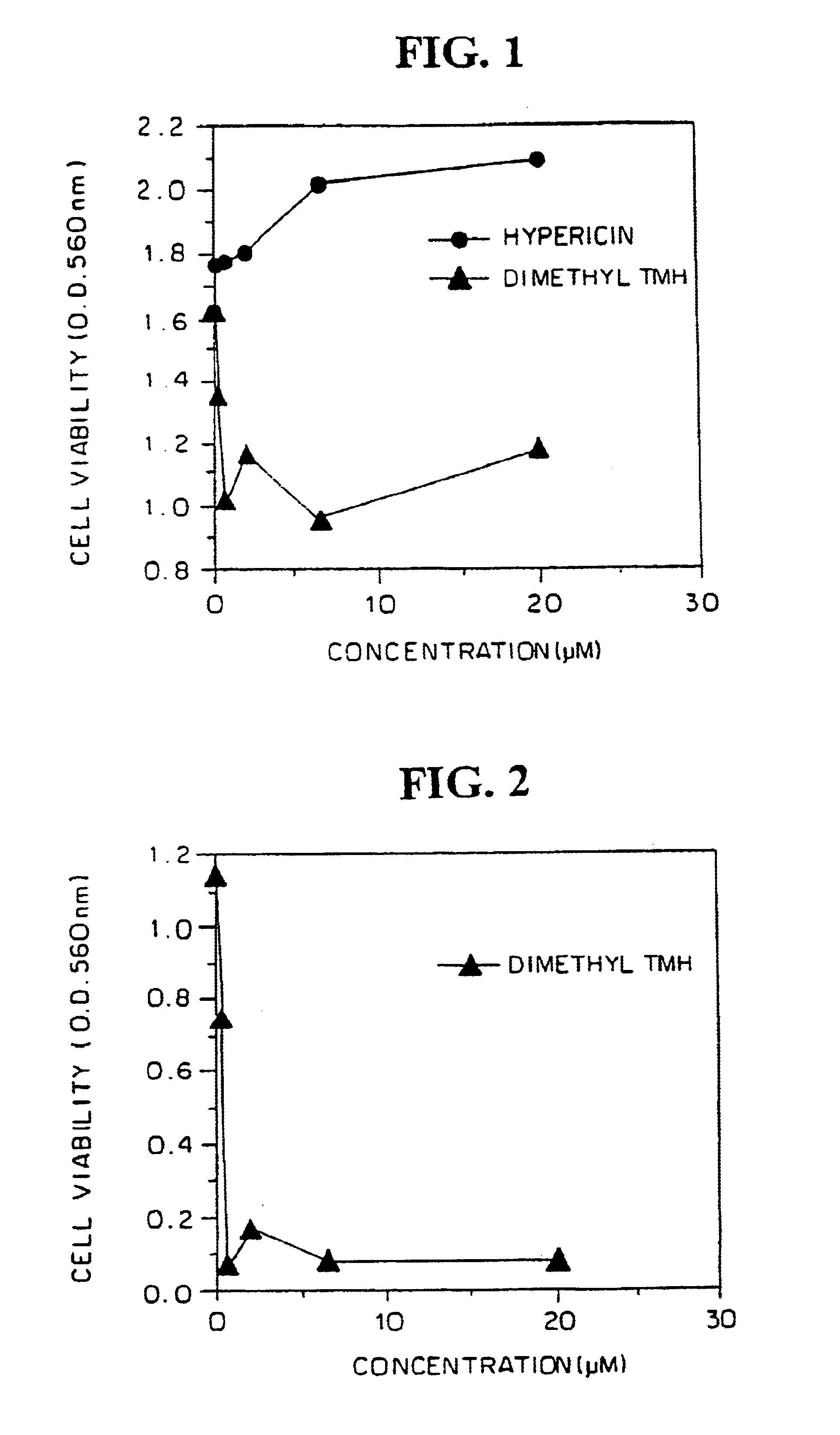
Characterization of biological tissues at a cellular level using red and far-red fluorescent dyes
InactiveUS20150104394A1Accurate administrationAccurate monitoringUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationFar-redMicroscopic scale
A method for observing the morphology of a biological tissue is disclosed. The method involves using a fluorescent dye on the biological tissue, where the fluorescent dye is selected from patent blue V, isosulfan blue, toluidine blue, hypericin, indocyanine green, MVAC, or doxorubicin, and using a microscopic linear or non-linear imaging system to form an image of the biological tissue, where the fluorescence of the dye reveals the morphology of the tissue at cellular scale.
Owner:INSTITUT GUSTAVE ROUSSY +1
Drug for preventing and treating gummosis
The invention discloses a drug for preventing and treating gummosis. The drug contains the following ingredients in parts by weight: 10-15 parts of 50% alkaloid-gardenoside, 8-12 parts of 70% Baume-degree lime-sulfur mixture, 10-15 parts of 70% mancozeb wettable powder, 4-8 parts of icariin, 4-8 parts of hypericin, 6-10 parts of forsythin, 4-8 parts of bupleurum roots and 6-10 parts of artemisia annua. The drug is simple in proportion and convenient in use, and the treatment effect on peach tree gummosis is good; shown by testing, the solution of a volume which is 400-600 times that of a mixture has an obvious inactivation effect on botryosphaeria and peach physalospora (belonging to ascomycotina) and has a good bactericidal effect.
Owner:QINGDAO MAOFENG ORGANIC VEGETABLE
Efficient hypericin synthesizing method initiated by monochromatic light
ActiveCN103274920ALight Response AdvantageShort reaction timeOrganic compound preparationQuinone preparationMicrowaveHypericin
The invention discloses an efficient hypericin synthesizing method initiated by monochromatic light. The method comprises the steps as follows: emodin is reduced to emodin anthrone under an acidic condition; the emodin anthrone is condensed to protohypericin through dimerization; and the protohypericin reacts under initiation of the monochromatic light to form the hypericin. According to the method, the dimerization reaction of the emodin anthrone takes a sodium hydroxide aqueous solution as a reaction medium and is performed in a microwave reactor, and has the characteristics of short reaction time, low temperature and high yield; in a light reaction, the monochromatic light with the wavelength of 575 nm is adopted to perform a light initiation reaction on a protohypericin acetone solution to prepare the hypericin, therefore, the reaction time is shortened greatly, and two steps of yields after column chromatography purification are as high as 96%; and additionally, the hypericin synthesizing method initiated by the monochromatic light provided by the invention has the advantages of convenience in operation, mild conditions, low synthetic cost and environment protection, and has bright development prospects.
Owner:NORTHWEST A & F UNIV
Method for purifying hypericin
The invention discloses technology for purifying hypericin. The technology is to take hypericum perforatum, overground parts provided with flowers of other plants containing the hypericin, and a crude extract or flower of the plants as raw materials, and to prepare hypericin purified products of which the content is higher than 98 percent after solvent extraction, separation and purification. The purified products can be used as chemical standard substances, reference substances or raw materials of preparation.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Process for preparing extract containing hypericin and flavon compounds
InactiveCN1392128ASolve solubilitySolve difficult to extractQuinone separation/purificationHypericinOrganic solvent
The present invention relates to the preparation process of extract containing hypericin and flavone compounds. The technological process includes extraction with polar organic solvent, biphase extraction and acid precipitation and through the simple process the extract containing total effective component not less than 50%, hypericin of 2-4 % and general flavone up to 50-90 % may be obtained. Inthe technological process, composite stabilizer is used, so that the extracted product may be maintained stably.
Owner:SUN YAT SEN UNIV
Production method of hyperforin perforatum extract
InactiveCN105596387AIncrease hypericin contentKeep active ingredientsPlant ingredientsHypericinBULK ACTIVE INGREDIENT
The invention belongs to the field of Chinese herbal medicines, and particularly relates to a production method of a hyperforin perforatum extract. The method is characterized by comprising the following steps: pretreatment of raw materials, extraction, leaching, concentration and the like. The hypericin content in an extract product is improved on the basis of ensuring active ingredients of various components of the product.
Owner:YAAN TAISHI BIOLOGICAL SCI & TECH CO LTD
Application of hyperforin to preparation of medicine for promoting browning of white fat and improving activity of brown fat
InactiveCN109044999AHigh energy consumptionLose weightOrganic active ingredientsMetabolism disorderBlood sugarLost Weight
The invention relates to the technical field of natural medicine chemicals and in particular relates to application of hyperforin to preparation of a medicine for promoting browning of white fat and improving the activity of brown fat. On the basis of action mechanisms of promoting browning of white fat and improving the activity of brown fat, the hyperforin has an effect of increasing energy consumption of a body to lose weight; in addition, the hyperforin has the effects of reducing blood sugar and blood grease levels, improving sugar resistance, improving insulin sensitivity and maintainingglycometabolism steadiness, and thus can be used as a medicine for relieving diabetes symptoms; in addition, the hyperforin can be also used for relieving or treating non-alcoholic fatty liver diseases. Therefore, the hyperforin, an extract with the hyperforin, or a preparation of the hyperforin has wide clinical application prospects in preventing or treating metabolic syndromes such as obesityand diabetes.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Clarification technology and quality control method of medicinal composition having acne treatment effect
InactiveCN107648369AAvoid overlappingReal qualityComponent separationPteridophyta/filicophyta medical ingredientsBeta-EcdysoneAdditive ingredient
The invention discloses a clarification technology and a quality control method of a medicinal composition having an acne treatment effect. The medicinal composition comprises Underleaf Pearl, Psammosilene tunicoides, Hypericum japonicum Thunb., Machilus wangchiana, Rhododendron primuliflorum Bur. et Franch., Root of Java Campanumoea, Hypericum sampsonii Hance, Lindera caudate, Calyx seu fructus Physalis, Indian kalimeris herb and Matteuccia struthiopteris. The clarification technology is concretely characterized in that medicinal composition extract is clarified by a type II ZTC 1+1 natural clarificant. The quality control method concretely comprises the following steps: carrying out thin-layer chromatography on the Underleaf Pearl, Psammosilene tunicoides, Lindera caudate, Calyx seu fructus Physalis and Indian kalimeris herb, and determining the content of vincetoxicoside, hyperoside, catechin, hypericin, lobetyolin and beta-ecdysone in the medicinal composition through high performance liquid chromatography. The clarification technology and the quality control method have the advantages of less device investment, simple operating process, overcoming of the defects of large alcohol consumption, tedious ethanol recovery, and complex determination process of multiple components in medicines of traditional alcohol precipitation methods, and improvement of the controllability ofthe quality standard of the medicinal composition.
Owner:GUANGZHOU QINGLAN BIOTECHNOLOGY CO LTD
Feed formula for improving immunities of piglets
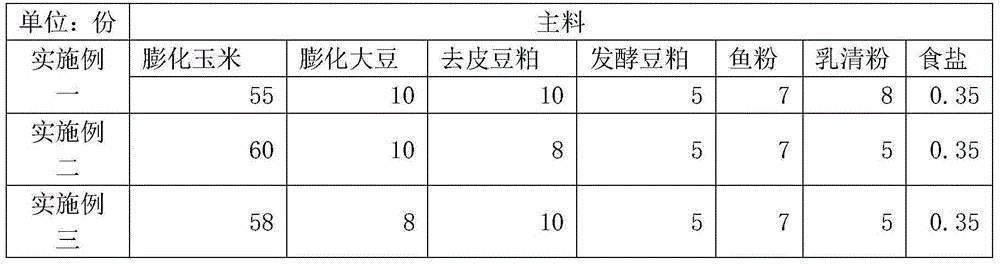
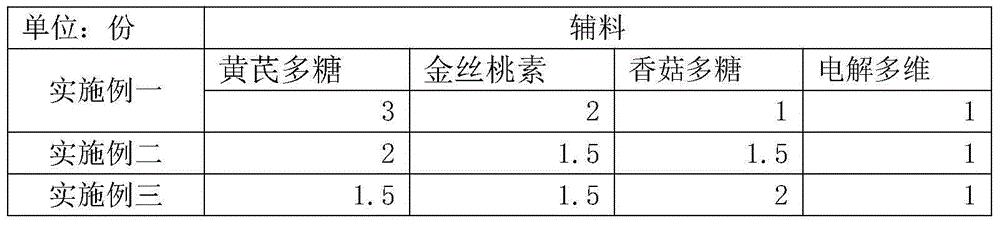
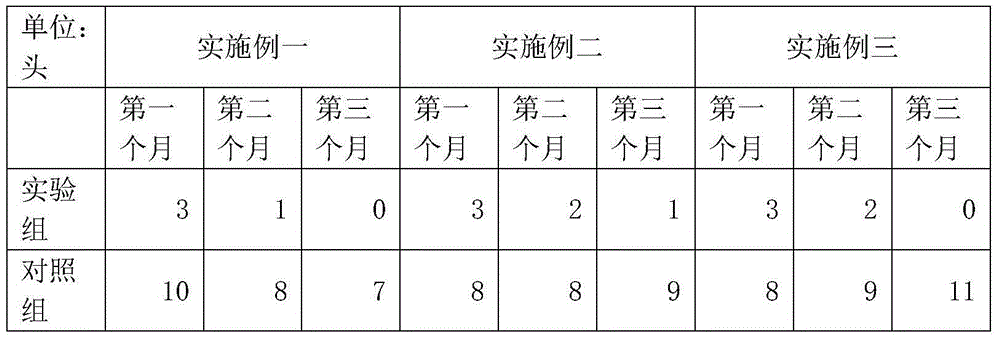
InactiveCN104431625AGood effectHydroxy compound active ingredientsInorganic active ingredientsDiseaseAnimal science
The invention discloses a feed formula for improving immunities of piglets; the feed formula comprises a main material and an auxiliary material; the main material comprises the following components in parts by weight: 30-40 parts of expanded corn, 25-30 parts of expanded soybean, 5-10 parts of peeled soybean meal, 5-10 parts of fermented soybean medal, 5-10 parts of fish powder, 5-10 parts of whey mist and 1-2 parts of salt; the auxiliary material comprises the following components in parts by weight: 2-4 parts of astragalus polysaccharide, 1-2 parts of hypericin, 1-2 parts of lentinan and 2-4 parts of electrolyzed multivitamins. The combination of the main material and the auxiliary material is capable of supplying nutritional substances such as protein and amino acid to the piglets, regulating the gastrointestinal systems of the piglets and improving digestive ability, so that the appetite of the piglets is increased and the effect of boosting health is achieved; moreover, the disease prevention abilities of the piglets are strengthened and the survival rate of the piglets is improved; the tonic medicine contains a large amount of amino acids and vitamins, so that the health of the piglets can be guaranteed and the nutritional value of pork is improved.
Owner:遵义市黔图农牧有限公司
Preparing method of hypericum perforatum extract
The invention relates to a preparation method of Hypericum Perforatum P.E, which is that extraction solvent containing effective components of hypericin and pseudohypericin is obtained by alcohol leaching extraction after the hypericum perforatum raw material is pretreated by immersing in water; then after the steps of heating, distilling, concentrating and extracting, the extract rich in effective components is obtained. The invention can be directly used in dry preparation of extract powder, can also be further separated to prepare hypericin and pseudohypericin with high purity.
Owner:周佳 +1
Radiosensitizer and preparation method thereof
InactiveCN104415348AReduce radiosensitivityImprove hypoxiaOrganic active ingredientsGenetic material ingredientsRadiation sensitizersHypericin
The invention relates to a radiosensitizer and a preparation method thereof, and the radiosensitizer can improve a radiation resistance problem of solid tumors; the radiosensitizer comprises 50%-80% of PLGA-PEI / hypericin nano colloidal particles, 5%-8% of HIF-1alpha-siRNA, and 12%-45% of hyaluronic acid, wherein the hyaluronic acid molecular weight can be any one of 11000, 6600 and 800. The preparation method comprises the steps of firstly preparing the PLGA-PEI / hypericin nano colloidal particles by an emulsion solvent evaporation method, then dropwise adding the HIF-1alpha-siRNA, and finally wrapping with HA. The preparation process is simple and easy to implement.
Owner:汪步海
Extraction method, extract and pharmaceutical use of hypericum perforatum extract
The invention discloses a method for preparing a hypericum perforatum extract. By the preparation method, a stable composition of the hypericum perforatum extract can be prepared, particularly a hypericin and hyperforin-containing composition. In addition, the invention also discloses a stable composition of the hypericum perforatum extract and use thereof for preparation of medicaments.
Owner:威海世纪博康海藻有限公司
Necrosis avid tracer agent
InactiveUS20070122340A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsHypericinPerylene
The present invention concerns to the use of phenanthro[1,10,9,8-opqra]perylene-7,14-dione derivatives, and more specifically hypericin or its derivatives, as necrosis or infarct specific agents. The phenanthro[1,10,9,8-opqra]perylene-7,14-dione derivatives can be labeled with a radionuclide, a radiopaque material or a material enhancing the effects magnetic resonance imaging.
Owner:K U LEUVEN RES & DEV
Synthesis method of hypericin
ActiveCN104193610AHigh yieldEliminate the purification processOrganic compound preparationQuinone separation/purificationHypericinSynthesis methods
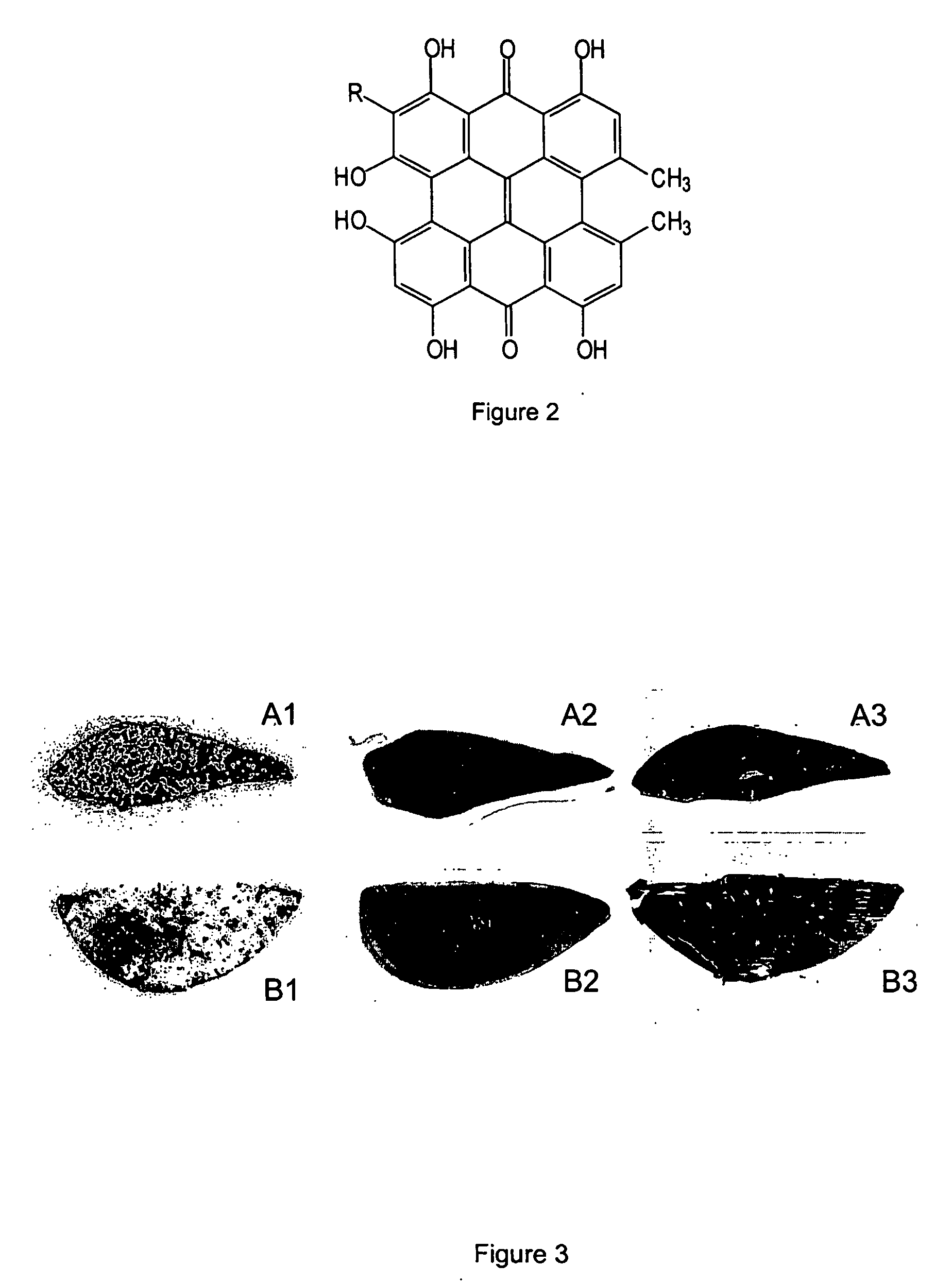
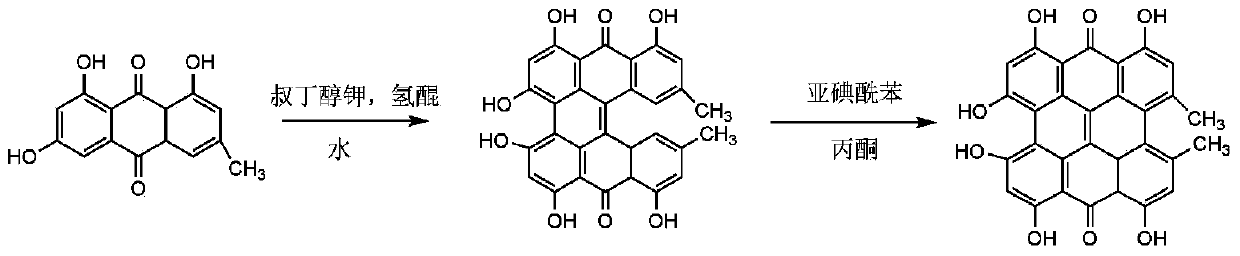
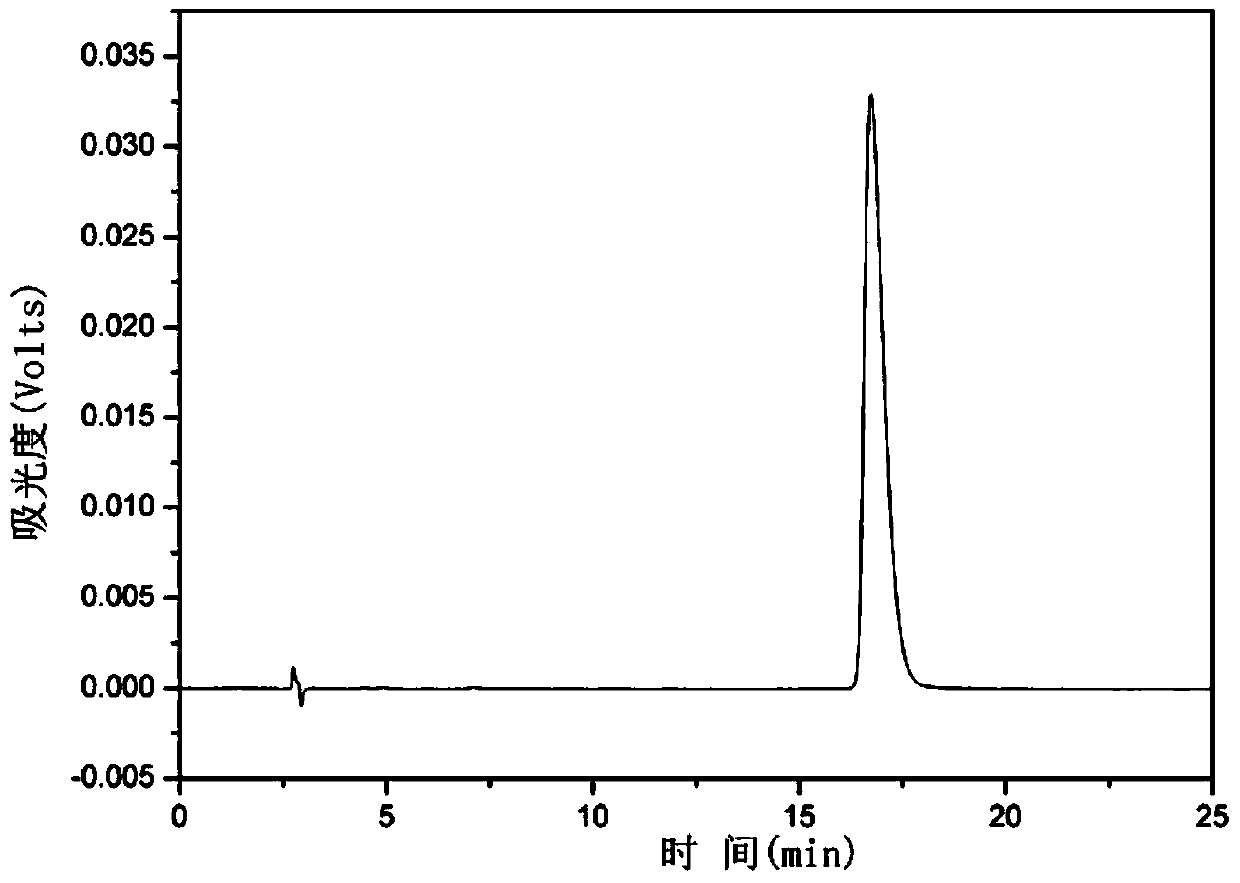
The invention discloses a synthesis method of hypericin. The synthesis method comprises the following steps of (a) dissolving emodin and tert-butanol salt into water, adding hydroquinone at a protective atmosphere, carrying out microwave irradiation, reacting at the temperature of 90-100 DEG C for 4-6h, then, transferring the product into a sealed vessel, continuing to react at the temperature of 120-140 DEG C for 8-12h, after finishing the reaction, adding an inorganic acid to regulate the pH value of the solution to 3-4, settling, filtering, washing, and drying, thereby obtaining raw hypericin at one step; and (b) dissolving the obtained raw hypericin and iodosobenzene substances into acetone, reacting at the temperature of 45-55 DEG C for 4-8h, filtering after finishing the reaction, collecting filtrate, carrying out evaporative concentration, washing, filtering and recrystallizing to obtain the hypericin. The hypericin obtained by using the synthesis method is high in purity and yield; and the synthesis method is simple in step, environment-friendly, reduced in cost and suitable for industrial production.
Owner:湖南世标健康科技有限公司
Medicinal composition for treating nevus flammeus and preparation method thereof
InactiveCN101744795ATo achieve both symptoms and root causesSignificant effectOrganic active ingredientsEnergy modified materialsSide effectHypericin
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com