Preparation method for hypericin albumin nanometer particles
A technology of albumin nanoparticles and hypericin is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, freeze-dried delivery, etc. Low content, hypericin is unstable to light and heat, etc., to achieve the effects of high drug loading and encapsulation efficiency, improved bioavailability, and surface roundness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
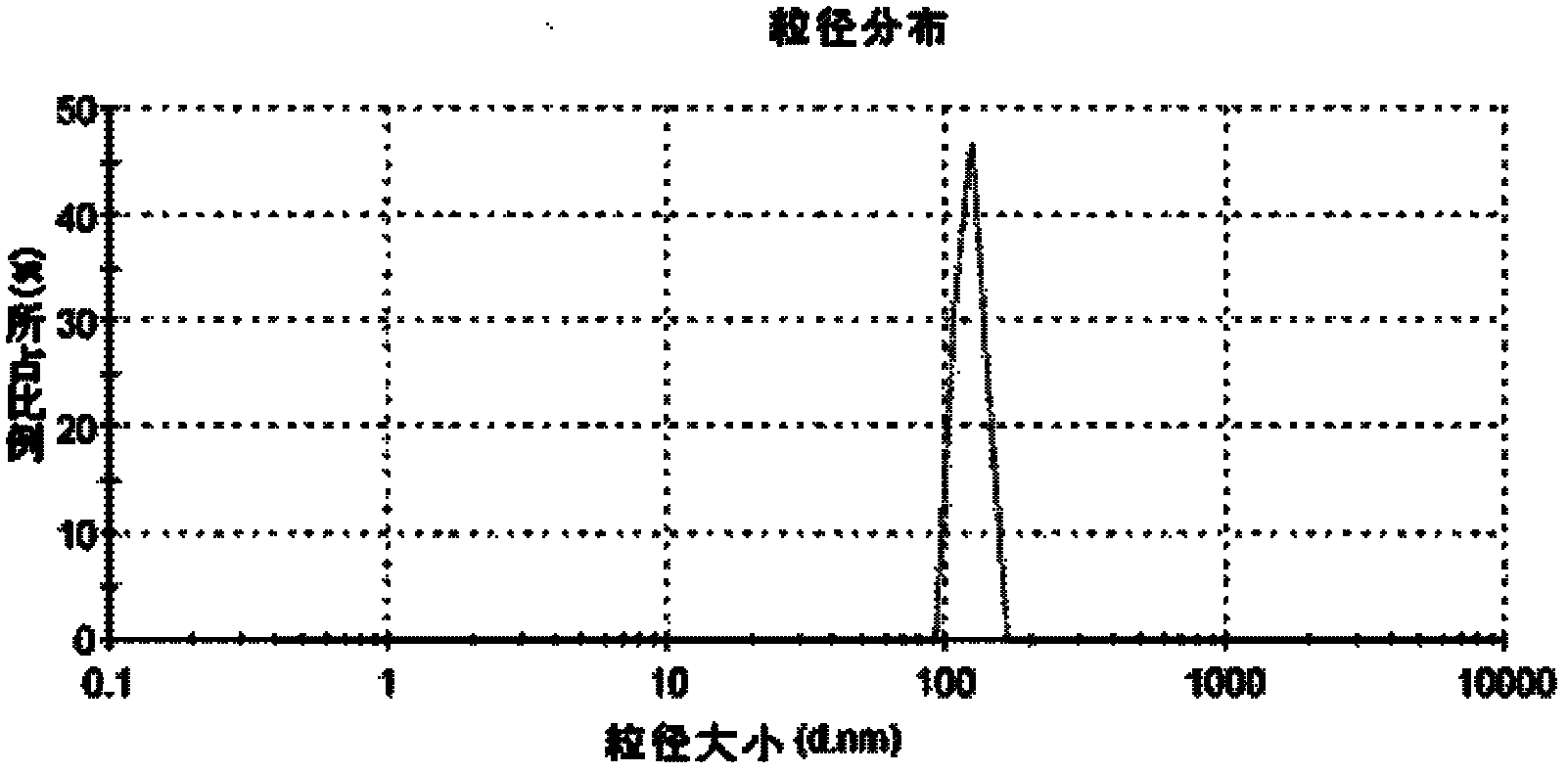
[0023] Accurately weigh 2.0 g of albumin and dissolve it in 98 ml of distilled water to make a carrier solution, weigh 4.0 g of HY and dissolve it in 96 ml of absolute ethanol to make an oil phase. rpm / min~1000 rpm / min), add 5 ml of oil phase dropwise to 50 ml carrier solution, fully emulsify and adjust PH=9, continue to add 20 ml of absolute ethanol dropwise at 0.5 ml / min, stir well for 2 h, solidified with glutaraldehyde for 24 h, centrifuged at 15,000 rpm / min for 15 min at high speed, washed 3 times, freeze-dried, and collected to obtain milky white hypericin albumin nanoparticles with an average particle size of 120 nm and a drug loading capacity of 4.12% , encapsulation efficiency 71.43%. The prepared hypericin albumin and ordinary hypericin were placed under fluorescent lamps, and their contents were measured on the 0th day, the 3rd day, the 5th day, and the 7th day respectively. It was found that the ordinary hypericin content The 3rd day decreased by 25.43%, the 5th d...
Embodiment 2
[0027] Accurately weigh 2.0 g of albumin and dissolve it in 98 ml of distilled water to make a carrier solution, weigh 4.0 g of HY and dissolve it in 96 ml of absolute ethanol to make an oil phase. rpm / min~1000 rpm / min), add 5 ml of the oil phase dropwise to 50 ml carrier solution, fully emulsify and adjust the PH=9, continue to add 20 ml of absolute ethanol dropwise at 1.0 ml / min, stir well for 2 h, solidified with glutaraldehyde for 24 h, centrifuged at 15,000 rpm / min for 15 min at high speed, washed 3 times, freeze-dried, and collected to obtain milky white hypericin albumin nanoparticles with an average particle size of 60 nm and a drug loading capacity of 4.54% , encapsulation efficiency 74.78%. The prepared hypericin albumin and ordinary hypericin were placed under fluorescent lamps, and their contents were measured on the 0th day, the 3rd day, the 5th day, and the 7th day respectively. It was found that the ordinary hypericin content The third day decreased by 26.17%, ...
Embodiment 3
[0029] Accurately weigh 2.0 g of albumin and dissolve it in 98 ml of distilled water to make a carrier solution, weigh 4.0 g of HY and dissolve it in 96 ml of absolute ethanol to make an oil phase. rpm / min~1000 rpm / min), add 5 ml of the oil phase dropwise to 50 ml carrier solution, fully emulsify and adjust PH=9, continue to add 20 ml of absolute ethanol dropwise at 2.0 ml / min, stir well for 2 h, solidified with glutaraldehyde for 24 h, centrifuged at 15,000 rpm / min for 15 min at high speed, washed 3 times, freeze-dried, and collected to obtain milky white hypericin albumin nanoparticles with an average particle size of 100 nm and a drug loading capacity of 4.23% , encapsulation efficiency 72.39%. The prepared hypericin albumin and ordinary hypericin were placed under fluorescent lamps, and their contents were measured on the 0th day, the 3rd day, the 5th day, and the 7th day respectively. It was found that the ordinary hypericin content The 3rd day decreased by 26.43%, the 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com