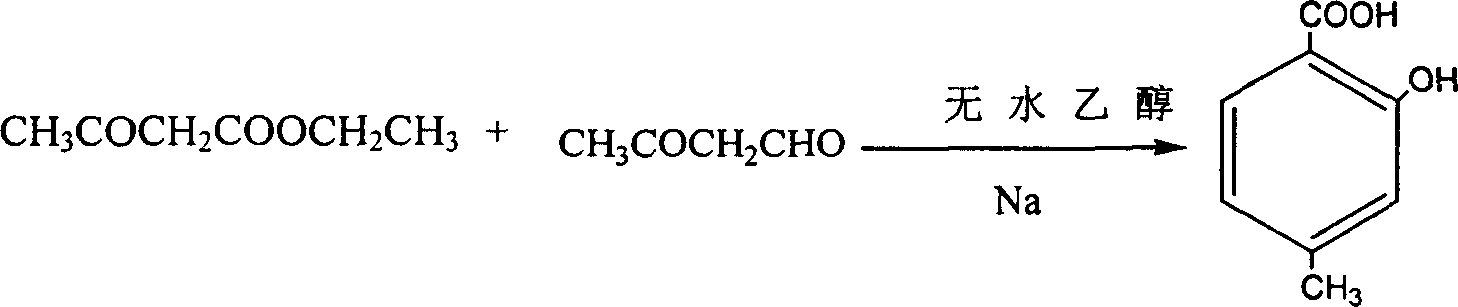Chemical synthesis process for hypericin and derivatives thereof
A kind of hypericin, a technology for chemical synthesis, applied in the field of chemical synthesis of compounds and derivatives thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
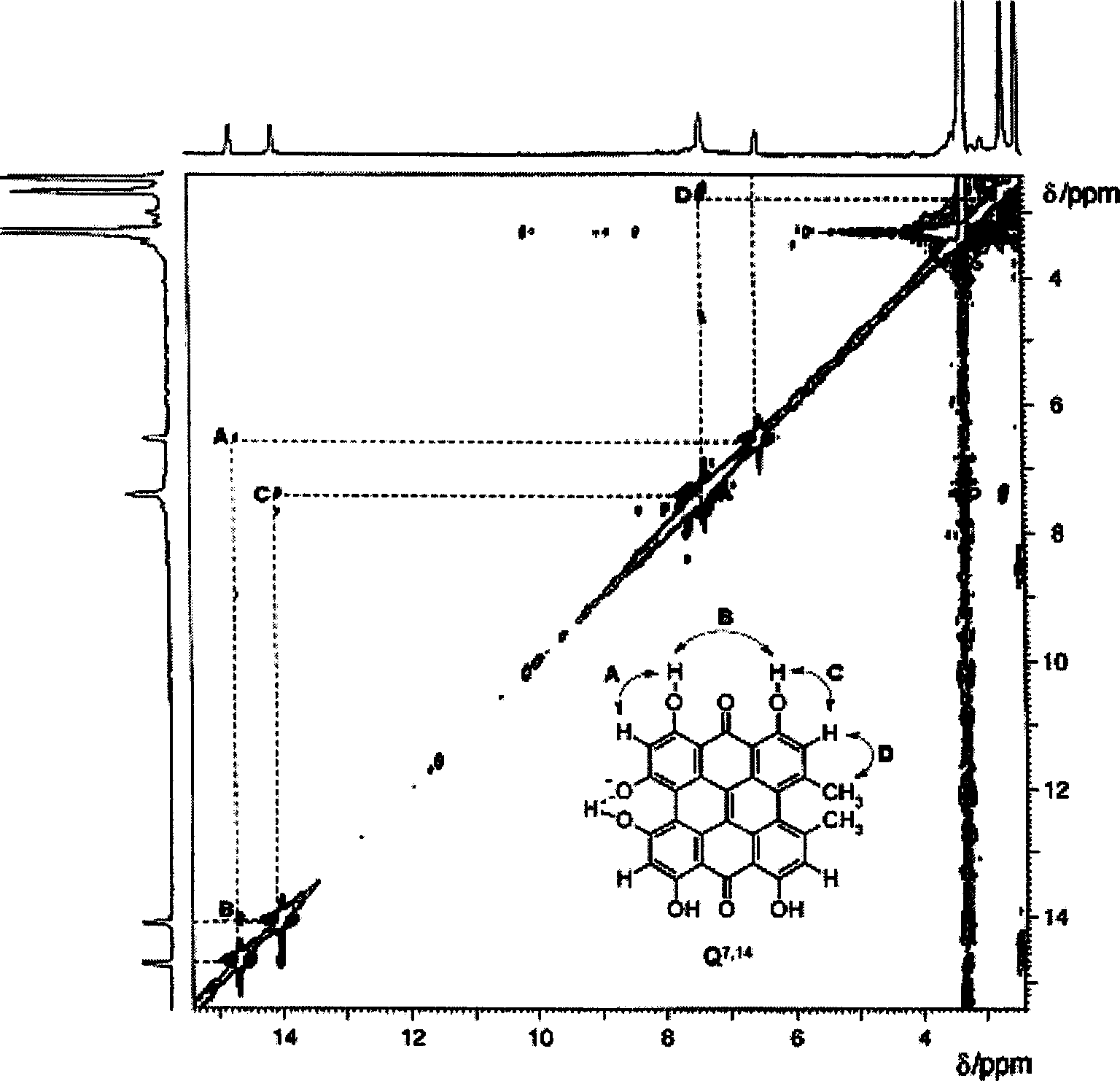
[0052] The synthesis of embodiment 1, hypericin
[0053] Now use the method of the present invention to artificially synthesize hypericin, and concrete process comprises the following steps:
[0054] 1) Synthesis of acetoacetaldehyde
[0055] The chemical equation for the synthesis of acetoacetaldehyde is as follows:
[0056] The specific method is: mix 18.5g (0.25mol) of ethyl formate and 17.4g (0.30mol) of acetone, slowly drop into 125mL of anhydrous ether containing 6g (0.15mol) of freshly cut sodium wire, and stir under the condition of avoiding water. , After the sodium wire reaction is complete, place it for 12-24h. The gray-yellow solid precipitate was filtered out, washed with anhydrous ether, and dried in vacuo to obtain 17.1 g of solid acetoacetaldehyde. After determination, the yield of acetoacetaldehyde was 80%.
[0057] 2) Synthesis of 2-hydroxyl-4-methylbenzoic acid
[0058] The chemical equation for the synthesis of 2-hydroxy-4-methylbenzoic acid is as fo...
Embodiment 2
[0082] Embodiment 2, the synthesis of hypericin heterocyclic derivatives
[0083]
[0084] Now taking the hypericin heterocyclic derivatives with the above chemical structural formula as an example, the synthetic method of hypericin heterocyclic derivatives is illustrated, that is, to synthesize its corresponding emodin heterocyclic derivatives first, and then obtain The target product of above-mentioned structural formula, concrete process comprises the following steps:
[0085] 1) Synthesis of 1,3,8-trihydroxy-6-benzimidazolone
[0086] Dissolve 166.6 mg of 1,3,8-trimethoxy-6-benzimidazole anthraquinone (0.402 mmol) in 30 mL of glacial acetic acid, and then add 725.6 mg of SnCl 2 .2H 2 The mass percent concentration of O (3.216mmol) is 45% of HBr15mL, filled with argon, refluxed at below 125°C for 1.3 hours, cooled, added to ice water, separated, and the residue was washed with water and vacuum-dried to obtain 130.2mg of product by weight. The yield was 90%. Instrumen...
Embodiment 3
[0089] Embodiment 3, the synthesis of hypericin sulfonic acid derivatives
[0090] R 1 = H or SO 3 H; R 2 = H or SO 3 h
[0091] Now taking the hypericin sulfonic acid derivatives with the above chemical structural formula as an example, the synthetic method of the hypericin sulfonic acid derivatives is illustrated, and the specific method is as follows:
[0092] 1. Synthesis of 2,5-disulfonic acid genserin
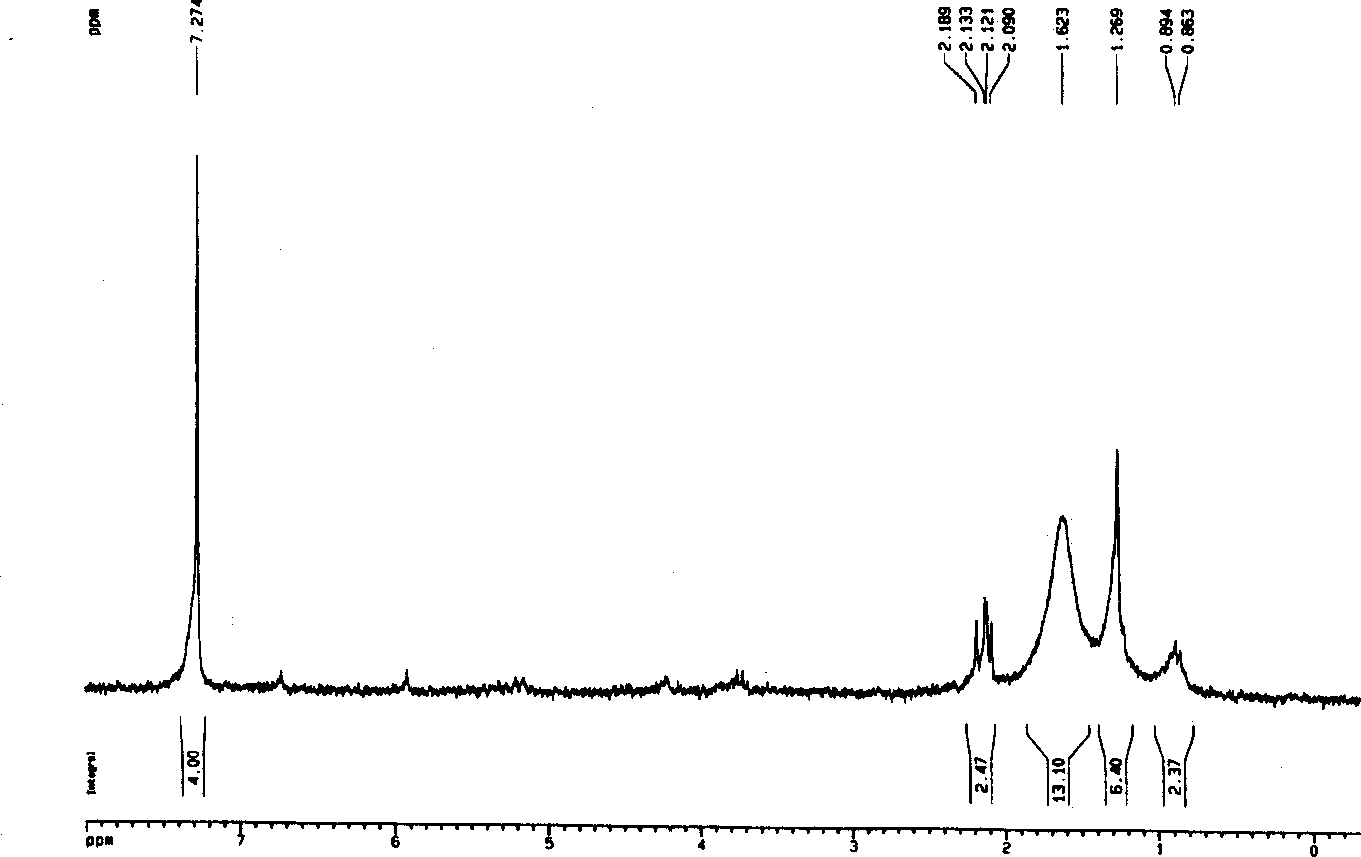
[0093] React 45mg of hypericin (0.089mmol) and 105mg of oleum (1.07mmol) at room temperature for 2 hours, dilute with ice water, add sodium chloride until saturated, centrifuge, wash the precipitate with cold water until neutral, and dry in vacuo. Eluted on Sephadex-LH O column, eluent is aqueous methanol (methanol: water=4: 1), obtains 2,5-disulfonic acid aristorin 8mg, productive rate is 10%; The product is carried out instrumental analysis , the result H 1 NMR (DMSO-d 6 , 500MHz, ppm): 15.00(s, 2H), 14.36(s, 2H), 13.51(s, 2H), 7.48(s, CH-9, 12), 2.74(s, 6H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com