Efficient hypericin synthesizing method initiated by monochromatic light
A technology for hypericin and original hypericin, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of quinones, etc., can solve the problems of cumbersome process of synthesizing hypericin, environmental pollution and high cost, and achieves The effect of reducing synthesis cost, high yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
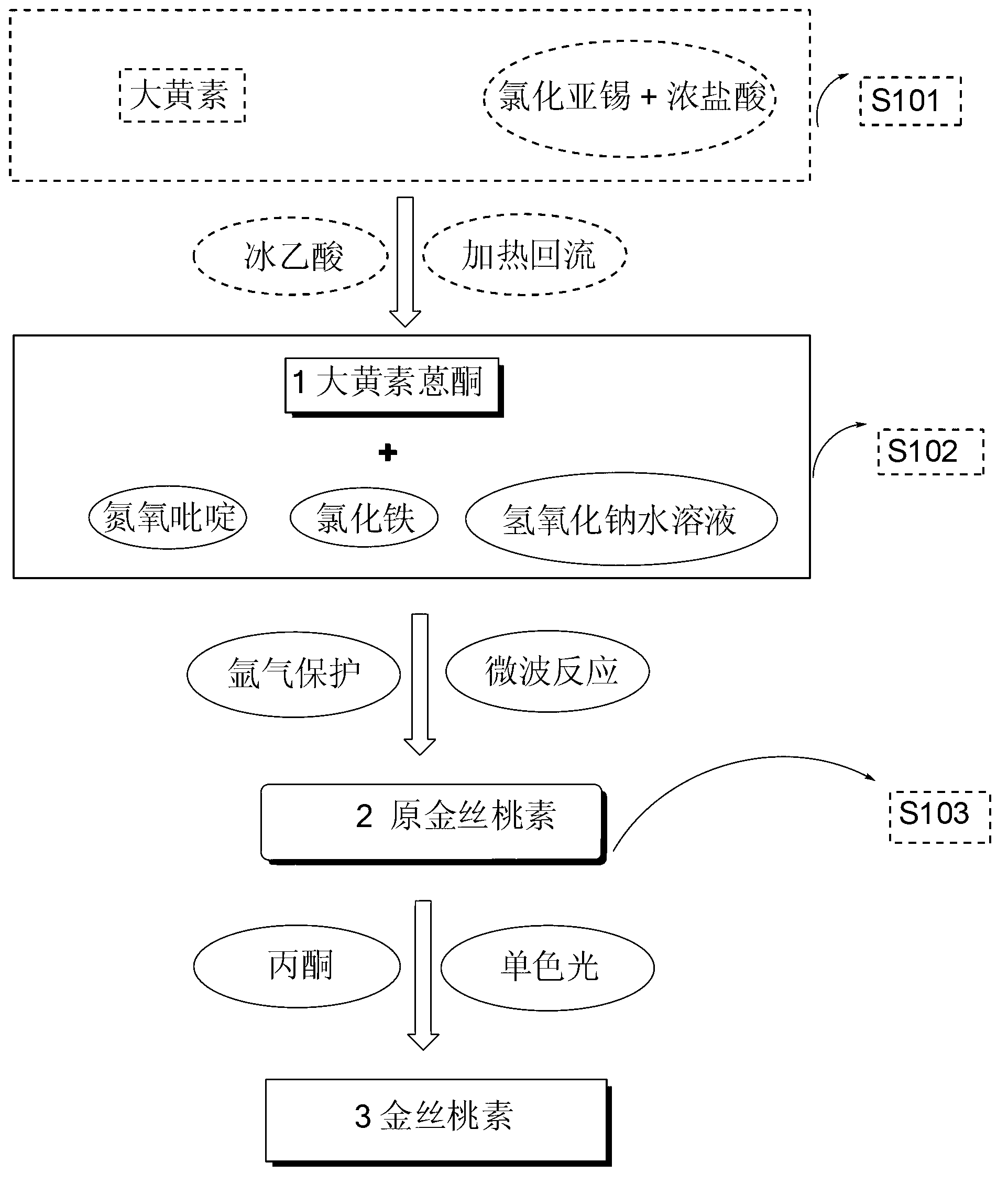
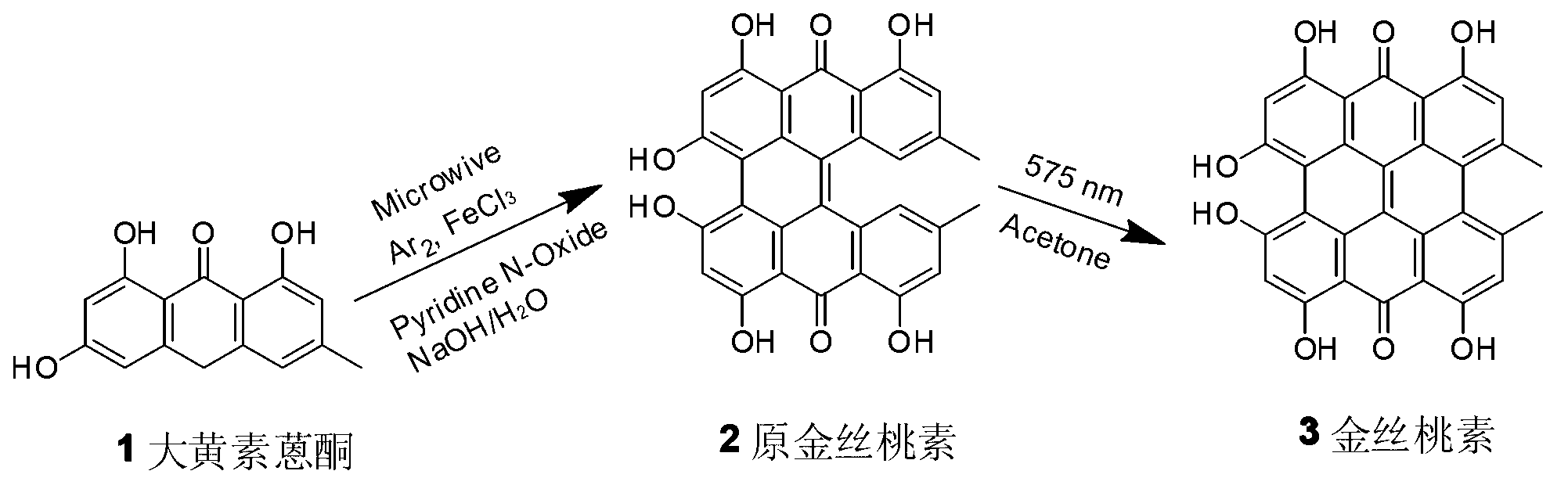
[0055] (1) Synthesis of protohypericin
[0056] In a 10mL microwave quartz tube, accurately weigh 130mg of emodin anthrone, 10mg of ferric chloride, 250mg of pyridine nitroxide, and 30mg of sodium hydroxide, then add 2mL of deionized water and blow argon into the quartz tube, and then put reaction in a microwave reactor. The temperature was set at 105° C., the microwave power was set at 10 W, and the reaction time was set at 70 min. After the reaction was completed, the reaction mixture was cooled to room temperature and neutralized with 3% hydrochloric acid solution, left to settle and filtered. The filter cake was washed thoroughly with deionized water and dried to obtain a black precipitate. The crude product was purified by a silica gel column to obtain 122 mg of a purple-black powder product with a yield of 96%.
[0057] (2) Synthesis of hypericin
[0058] Accurately weigh 127mg of original hypericin in a 125mL conical flask and dissolve it in 100mL acetone. Irradiate ...
Embodiment 2
[0060] (1) Synthesis of protohypericin
[0061] In a 35mL quartz tube, accurately weigh 1.28g of emodin anthrone, 107mg of ferric chloride, 301mg of sodium hydroxide, 2.51g of nitrogen pyridine, and add H 2 O20mL. After blowing in argon gas, it was put into a microwave reactor for reaction. The temperature was set at 105° C., the microwave power was set at 10 W, and the reaction time was set at 70 min. After the reaction, according to the scheme one post-processing to obtain 1.16g of purple black prohypericin powder, the yield is 92%
[0062] (2) Synthesis of hypericin
[0063] In a 250mL Erlenmeyer flask, accurately weigh 1.00g of original hypericin, and add 200mL of acetone into it. Stir under the protection of argon, irradiate the reaction with 575nm monochromatic light, follow TLC, and the reaction is complete in 3 hours. Acetone was distilled off under reduced pressure to obtain a crude product, which was purified by a silica gel column to obtain 895 mg of black hype...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com