Synthesis method of hypericin
A technology of hypericin and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of multiple column passes, high cost, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
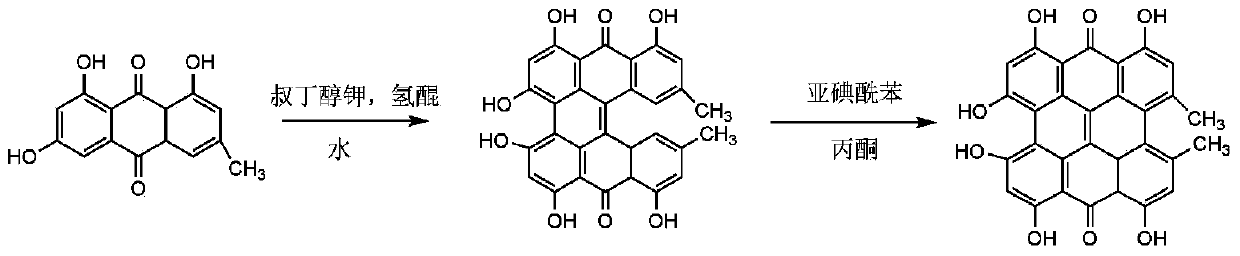
[0029] (1) Synthesis of protohypericin from emodin
[0030] Emodin 270 mg, potassium tert-butoxide 347 mg, distilled water 30 mL, hydroquinone 230 mg, the above reactants were added to a 50 mL round bottom flask, and the N 2 , 200w microwave irradiation reaction at 95°C for 4-6h. After the reaction was initiated, the solution was quickly transferred to a reaction kettle and placed in an oven at 120°C for 8 h. After opening the reaction kettle, 3% by mass hydrochloric acid was added dropwise to adjust the solution to pH=3, the precipitate was filtered out, washed with water until neutral, and vacuum-dried to obtain prohypericin as a black powder solid with a yield of 90%.
[0031] (2) Protohypericin conversion hypericin
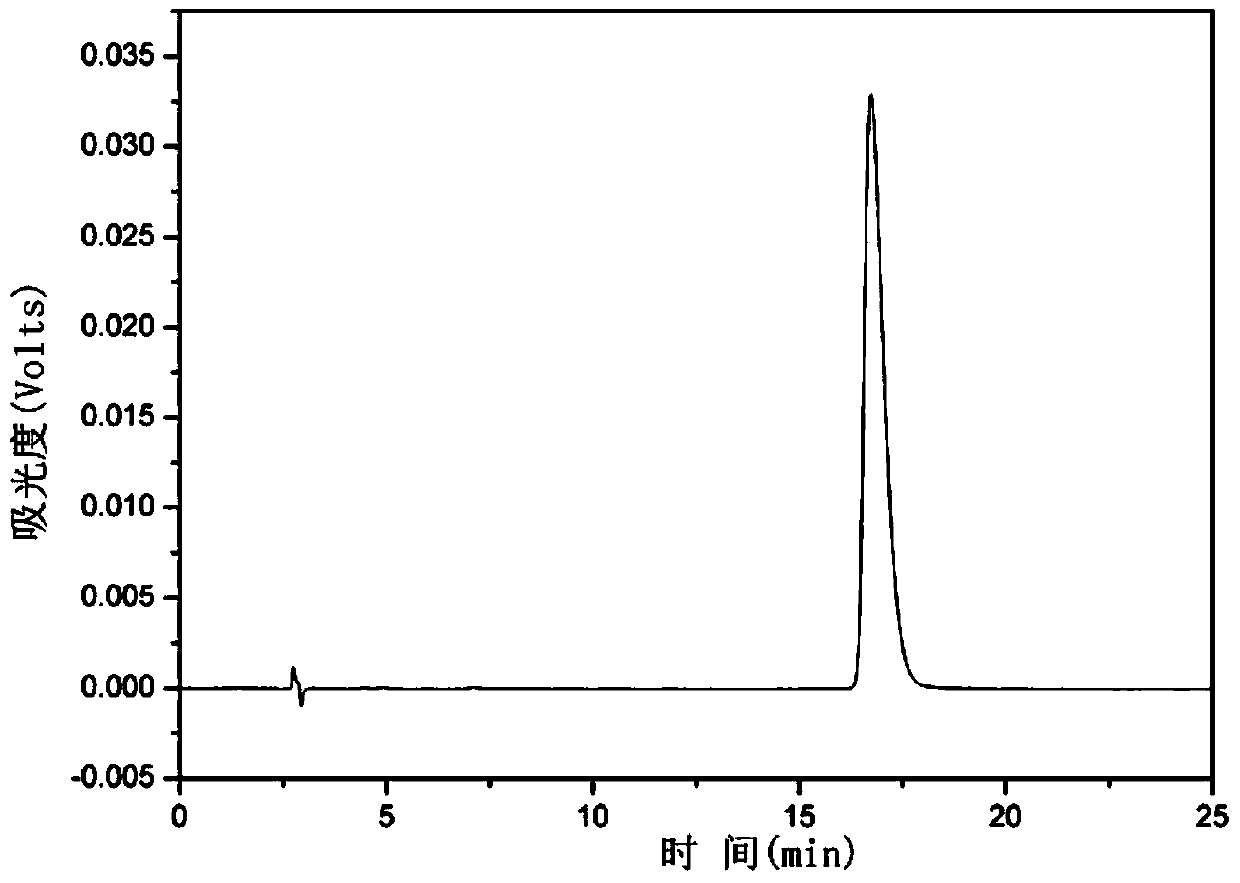
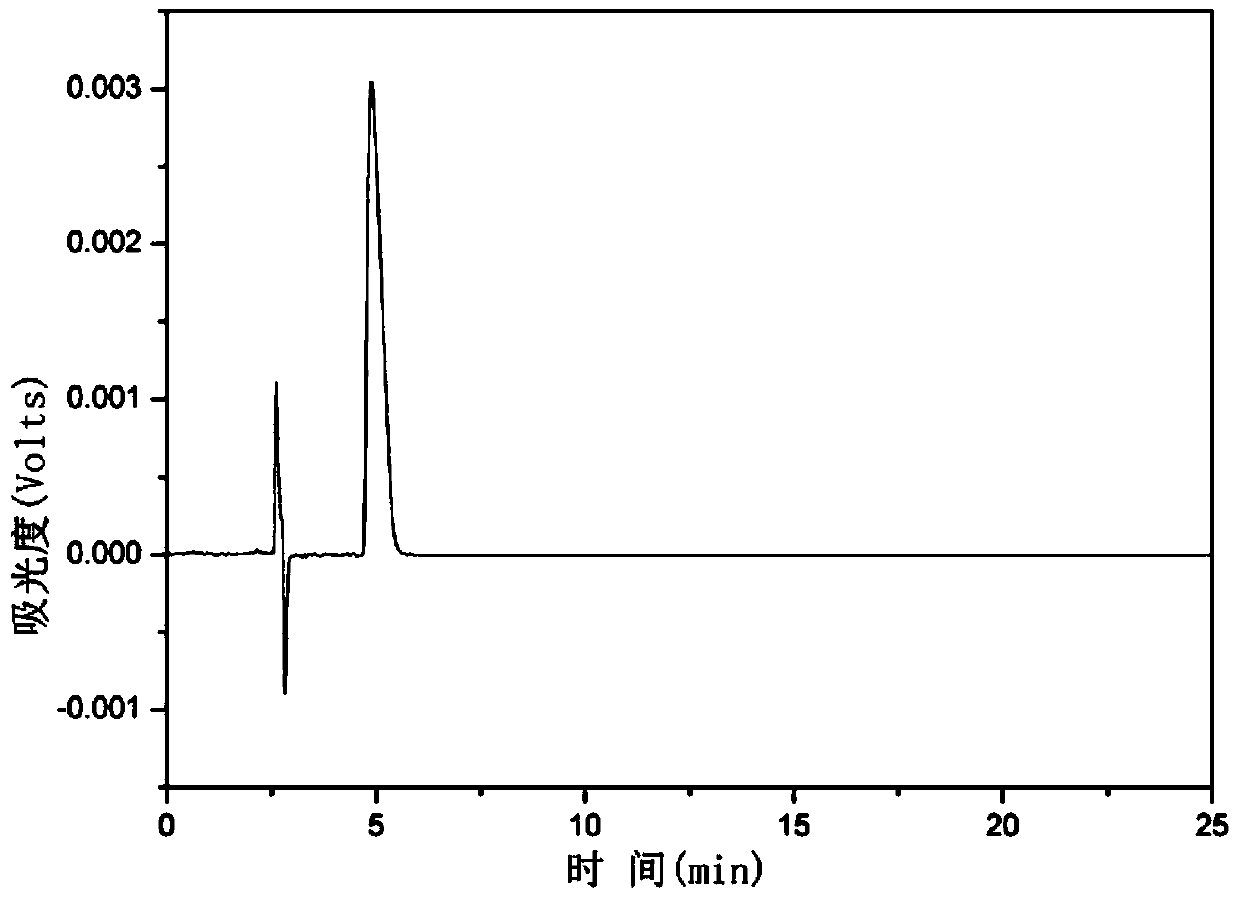
[0032] Weigh 100 mg of the original hypericin obtained in step (1) and add 44 mg of iodosobenzene, dissolve in 100 mL of acetone, heat at 45°C while stirring, react for 4 hours, filter, collect the filtrate, and remove the acetone by rotary evaporation . Th...
Embodiment 2
[0034] (1) Synthesis of protohypericin from emodin
[0035] Emodin 270 mg, sodium tert-butoxide 300 mg, distilled water 30 mL, hydroquinone 230 mg, the above reactants were added to a 50 mL round bottom flask, and a three-way pipe was connected to N 2 ,, 300w microwave irradiation reaction under the condition of 95 ℃ for 4 ~ 6h, after the reaction was initiated, the solution was quickly transferred to the reaction kettle, and placed in a 130 ℃ oven for 10h. After the reactor was opened, 3% sulfuric acid was added dropwise to adjust the solution to pH = 3, the precipitate was filtered off, washed with water until neutral, and vacuum-dried to obtain prohypericin as a black powder solid with a yield of 89%.
[0036] (2) Protohypericin conversion hypericin
[0037] Weigh 100 mg of the original hypericin obtained in step (1), add 30 mg of iodosobenzene, dissolve in 150 mL of acetone, heat at 50°C while stirring, react for 6 hours, filter, collect the filtrate, and remove the aceto...
Embodiment 3
[0039] (1) Synthesis of protohypericin from emodin
[0040] Emodin 270mg, Potassium tert-butoxide 350mg, distilled water 30mL, hydroquinone 330mg, add the above reactants into a 50 mL round bottom flask, connect N with a three-way tube 2 , 400w microwave irradiation reaction at 100°C for 4-6h, after the reaction was initiated, the solution was quickly transferred to a reaction kettle and placed in an oven at 140°C for 12h. After opening the reaction kettle, add 3% hydrochloric acid drop by drop, adjust the solution to pH = 3, filter out the precipitate, wash the precipitate with water until neutral, and dry it in vacuum to obtain a black powdery solid protohypericin with a yield of 90%. .
[0041](2) Protohypericin conversion hypericin
[0042] Weigh 100 mg of the original St. John's Wort obtained in step (1), add 22 mg of iodosobenzene, dissolve in 200 mL of acetone, heat at 55 ° C while stirring, and after 8 hours of reaction, filter, collect the filtrate, and remove the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com