Method for synthesizing hypericin
A synthetic method and technology of hypericin, applied in chemical instruments and methods, preparation of organic compounds, preparation of quinones, etc., to achieve the effects of high yield, mild reaction conditions and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
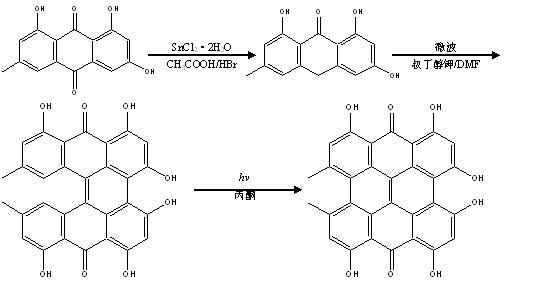
[0014] (one). Synthesis of Emodin Anthrone
[0015] Accurately weigh 1.78 g of emodin and 200 ml of glacial acetic acid in a four-neck flask, stir under nitrogen protection, heat up to reflux, and then add 5.86 g of SnCl 2 2H 2 0, and in the whole process of the reaction, slowly dropwise add 30 ml of hydrobromic acid with a mass fraction of 48%. After reacting for 6 h, the stirring and nitrogen protection were stopped. After the solution was cooled, it was suction-filtered, and the filter cake was washed with distilled water until neutral, and dried to obtain 1.48 g of a light yellow powder with a yield of 96.1% and a melting point of 254°C-258°C. During the reaction process, the reaction was tracked by thin-layer chromatography, and the developer used was a mixed solution of benzene-ethanol (v / v, 8:2). IR (potassium bromide compressed tablets), v / cm -1 : 1620 cm -1 ; MS, m / z 255 (M — ); 1 H-NMR (DMSO-d6): δH: 2.33 (s, 3H, Me), 4.31 (s, 2H, CH 2 ), 6.22(d, 1H, J = ...
Embodiment 2
[0021] (one). Synthesis of Emodin Anthrone
[0022] Different from Example 1, the amount of emodin used is 0.83 g, glacial acetic acid is 150 ml, SnCl 2 2H 2 O 3.25 g, hydrobromic acid 20 ml. The reaction time was 4.5 h, and the yield was 86.2%.
[0023] All the other steps are the same as in Example 1.
[0024] (two). Synthesis of Protohypericin
[0025] The difference from Example 1 is that the amount of emodin anthrone used is 1.88 g, potassium tert-butoxide is 0.05 g, and dimethylformamide is 5 ml. The reaction temperature was 130 ℃, the microwave power was 4 W, the reaction was 0.6 h, and the yield was 73.9%.
[0026] All the other steps are the same as in Example 1.
[0027] (three). Synthesis of hypericin
[0028] The difference from Example 1 is that the amount of protohypericin used is 0.88 g, acetone is 500 ml, and n-hexane is 100 ml. The power of the halogen lamp used was 200 W, and the obtained yield was 82.5%.
[0029] All the other steps are the same ...
Embodiment 3
[0031] (one). Synthesis of Emodin Anthrone
[0032] Different from Example 1, the amount of emodin used is 3.55 g, glacial acetic acid is 500 ml, SnCl 2 2H 2 O 12.65 g, hydrobromic acid 100 ml. The reaction time was 5 h, and the yield was 78.5%.
[0033] All the other steps are the same as in Example 1.
[0034] (two). Synthesis of Protohypericin
[0035] The difference from Example 1 is that the amount of emodin anthrone used is 3.22 g, potassium tert-butoxide is 0.76 g, and dimethylformamide is 15 ml. The reaction temperature was 180 ℃, the microwave power was 10 W, the reaction was 0.5 h, and the yield was 68.3%.
[0036] All the other steps are the same as in Example 1.
[0037] (three). Synthesis of hypericin
[0038] The difference from Example 1 is that the amount of protohypericin used is 2.33 g, acetone is 2000 ml, and n-hexane is 200 ml. The power of the halogen lamp used was 100 W, and the yield was 73.2%.
[0039] All the other steps are the same as in Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com