Macrocyclic and cage-like molecules based on biphenylarene and derivatives of macrocyclic and cage-like molecules as well as synthetic methods and applications of macrocyclic and cage-like molecules and derivatives
A technology for caged compounds and macrocyclic compounds, which is applied in the field of macrocyclic and caged molecules and derivative compounds and their synthesis, and can solve the problems of few modification sites, cumbersome synthetic routes, and low yields.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Synthesis and derivatization of biphenyl arene macrocycle and molecular cage, using dibromo or tribromo raw materials, carry out suzuki coupling reaction to obtain bis-(2, 4-dialkoxyphenyl) arene monomer (named M1-M27), and then use it and aldehydes as raw materials, halogenated alkanes as solvents, and Lewis acids as catalysts to obtain biphenyl aromatic macrocycles and molecular cages. Through further derivatization and modification, water-soluble Macrocyclic aromatic hydrocarbons and molecular cages (named 1-41). The synthesis and derivatization of biphenyl arene macrocycle and molecular cage include the following steps:
[0169] Synthesis of step 1 biphenyl arene monomer compound;
[0170] Step 2 is based on the synthesis of macrocycles and molecular cages of biphenyl arenes;
[0171] Step 3 is the derivatization of biphenyl arene macrocycle and molecular cage.
[0172] The synthetic method of step 1 biphenyl arene monomer compound is as follows:
[0173] [1] Pr...
Embodiment 2
[0439] The macrocyclic and caged and derived compounds of biphenyl arene are used in materials, environment and biology.
[0440] (1) Application of biphenyl aromatic hydrocarbon macrocyclic compounds and molecular cages
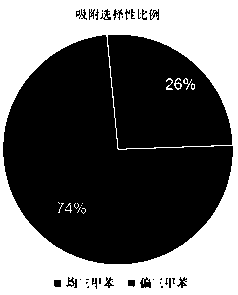
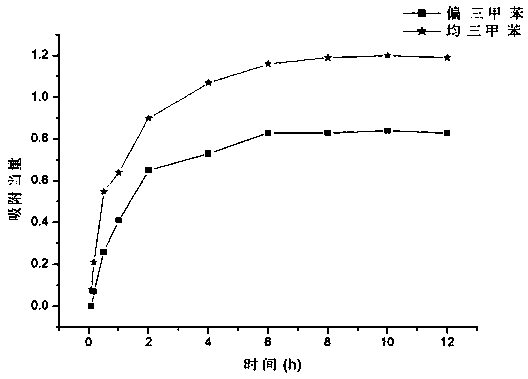
[0441] 【1】Application of macrocyclic aromatic hydrocarbons as adsorption and separation materials
[0442] The application of trimethylbenzene isomers is very extensive. However, because the boiling points between different trimethylbenzene isomers are very close, it is extremely difficult to separate the trimethylbenzene isomers in industry. The ring has a unique cavity structure, so it can selectively adsorb and separate trimethylbenzene isomers.
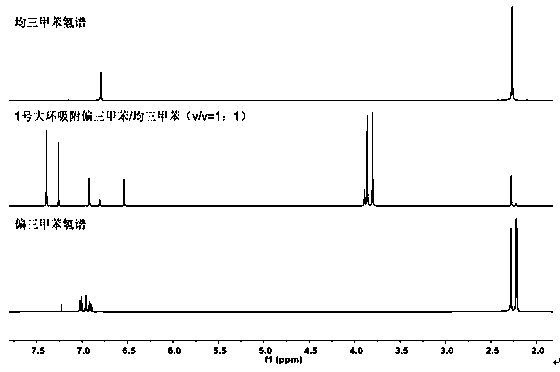
[0443] We put the activated single crystal of No. 1 macrocyclic compound into a vacuum oven for 8 hours, put the material in the mixed vapor of saturated mesitylene: mesitylene for 12 hours, dissolve it in deuterated chloroform and carry out Hydrogen spectrum characterization. The hydrogen spectrum shows that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com