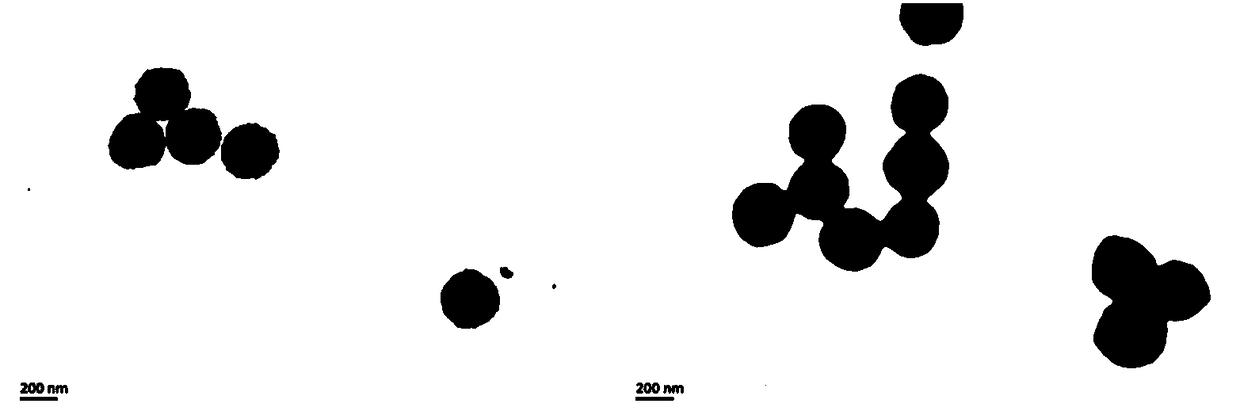
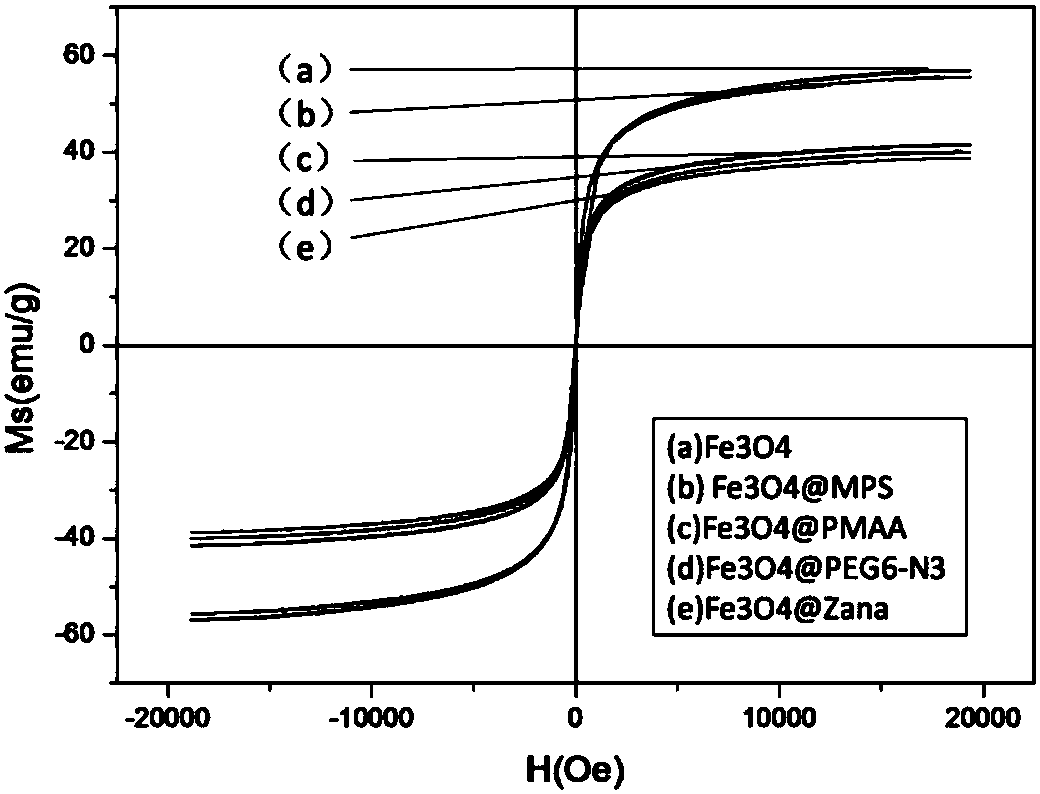
Zanamivir-magnetic nanoparticle conjugate, preparation method thereof and application
A technology of magnetic nanoparticles and zanamivir, applied in the field of zanamivir-magnetic nanoparticle conjugate and its preparation, can solve the problem of high cost of antibody-magnetic nanoparticles, long period of antibody preparation and limited applicability and other problems, to achieve the effect of being conducive to preservation and application, simple and convenient preparation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
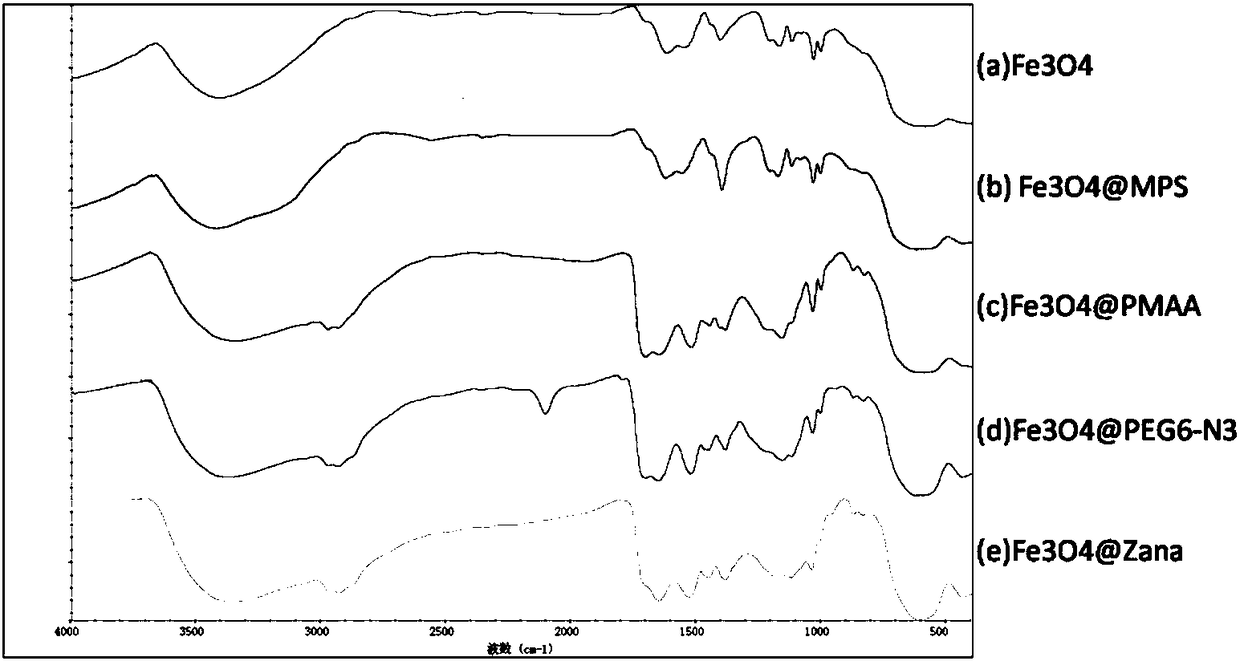
Image
Examples
Embodiment
[0207] Unless otherwise specified, the methods used in the present invention below are conventional methods; the materials and reagents used can be obtained from commercial sources.
[0208] Reagents used below
[0209] Tris-HCl buffer solution (100 mM, pH 8.0): Tris was dissolved in water at a final concentration of 12.1 g / L, and the pH was adjusted to 8.0 with HCl.
[0210] PBS buffer (10 mM, pH 7.4): NaH 2 PO 4 0.24g / L, Na 2 HPO 4 1.42g / L, KCl 0.2g / L and NaCl 8.0g / L, the solvent is water.
[0211] PBST buffer (10 mM, pH 7.4): NaH 2 PO 4 0.24g / L, Na 2 HPO 4 1.42g / L, KCl 0.2g / L, NaCl 8.0g / L and Tween-20 0.5ml / L, the solvent is water.
[0212] TBS buffer (10mM, pH 7.4): Tris 3g / L, KCl 0.2g / L and NaCl 8.0g / L, solvent is water.
[0213] TBST buffer (10mM, pH 7.4): Tris 3g / L, KCl 0.2g / L, NaCl 8.0g / L and Tween-200.5ml / L, solvent is water.
[0214] 2-(4-morpholine)ethanesulfonic acid (MES, 50nM, pH 5.0) activation buffer, MES dissolved in water, the final concentra...
preparation example 1
[0221] Preparation Example 1 N 3 CH 2 (CH 2 OCH 2 ) 5 CH 2 NH 2 Synthesis
[0222] The compound synthesized in this preparation example is N 3 CH 2 (CH 2 OCH 2 ) 5 CH 2 NH 2 (i.e. the compound shown in formula X-3, m=5), its synthetic route is as follows:
[0223]
[0224] The synthesis of compound 1: Hexapolyethylene glycol (1.41g, 5mmol) was dissolved in triethylamine (3.5mL, 25mmol) and dry dichloromethane (100mL), ice-bathed, p-toluenesulfonyl chloride (2.4 g, 15 mmol) was stirred at room temperature for 24 hours. TLC (EtOAc) detected the completion of the reaction. Add dichloromethane (200mL) to dilute, successively wash with 1M HCl﹑saturated NaHCO 3 and saturated NaCl extraction, with anhydrous NaCl 2 SO 4 The organic phase is dried. Filter to remove anhydrous Na 2 SO 4 Afterwards, the solvent of the organic phase was evaporated to dryness under reduced pressure. The resulting mixture was separated and purified by silica gel column chromatography...
preparation example 2
[0227] Preparation Example 2 Synthesis of Zanamivir Modified with Alkyne Group
[0228] The zanamivir modified with alkynyl group synthesized in this preparation example is compound 5, (i.e. in the compound shown in formula X-2, m=6), and its synthesis route is as follows:
[0229]
[0230] Compound 4 was prepared according to the method disclosed in CN103819665B. 156mg of compound 4 (0.167mmol) was put into a 25mL reaction flask, dissolved in 10ml of methanol, the pH of the reaction solution was adjusted to 13 with 1M NaOH aqueous solution, and stirred at room temperature for 3 hours. TLC monitors reaction process, adds acidic resin Dowex 50 (H + ) to neutralize and adjust the pH of the reaction solution to about 7. After being filtered and spin-dried, the obtained crude product was dissolved in a mixed solution of trifluoroacetic acid and dichloromethane (trifluoroacetic acid:dichloromethane=1:1), and stirred at room temperature for 3 hours. The progress of the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com