A class of macrocyclic and cage-like molecules and derivatives based on biphenyl aromatic hydrocarbons and their synthesis methods and applications
A technology for caged compounds and macrocyclic compounds, which is applied in the field of macrocyclic and caged molecules, derivative compounds and their synthesis, and can solve the problems of few modification sites, low yield, and complicated synthesis routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Synthesis and derivatization of biphenyl aromatic macrocycles and molecular cages, with dibromo or tribromo raw materials, carry out suzuki coupling reaction to obtain bis-(2, 4-dialkoxyphenyl) aromatic monomers (named It is M1-M27), then it and aldehydes are used as raw materials, halogenated alkanes are used as solvents, Lewis acids are used as catalysts, and the reaction obtains biphenyl aromatic macrocycles and molecular cages. Through further derivatization and modification, water-soluble compounds can be obtained Macrocyclic aromatic hydrocarbons and molecular cages (named 1-41). The synthesis and derivatization of biphenyl aromatic macrocycles and molecular cages include the following steps:
[0169] The synthesis of step 1 biphenyl aromatic hydrocarbon monomer compound;
[0170] Step 2 is based on the synthesis of macrocycles and molecular cages of biphenyl aromatics;
[0171] Step 3: Derivatization of biphenyl aromatic macrocycles and molecular cages.
[017...
Embodiment 2
[0439] Macrocyclic and caged and derived compounds of biphenyl aromatic hydrocarbons for material, environmental, and biological applications.
[0440] (1) Application of biphenyl aromatic macrocyclic compounds and molecular cages
[0441] [1] Application of macrocyclic aromatic hydrocarbons as adsorption separation materials
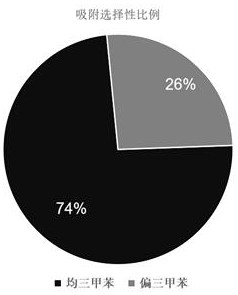
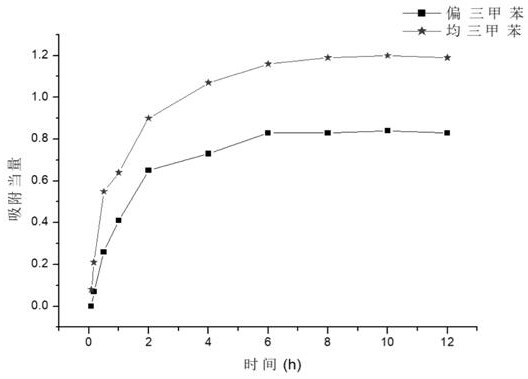
[0442] Trimethylbenzene isomers are widely used. However, because the boiling points of different trimethylbenzene isomers are very close, the separation of trimethylbenzene isomers is extremely difficult in industry. Due to the large supramolecular size of our synthesized The ring has a unique cavity structure, so it can selectively adsorb and separate trimethylbenzene isomers.
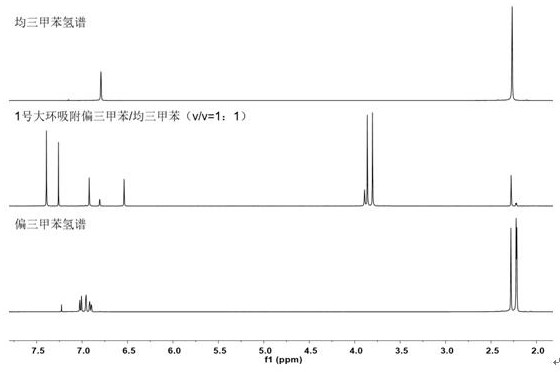
[0443] We put the activated single crystal of macrocyclic compound No. 1 into a vacuum oven for 8 hours, and put the material in the mixed vapor of saturated mesitylene: mesitylene for adsorption for 12 hours, and then dissolved it in deuterated chloroform. Hydrogen spectrum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com