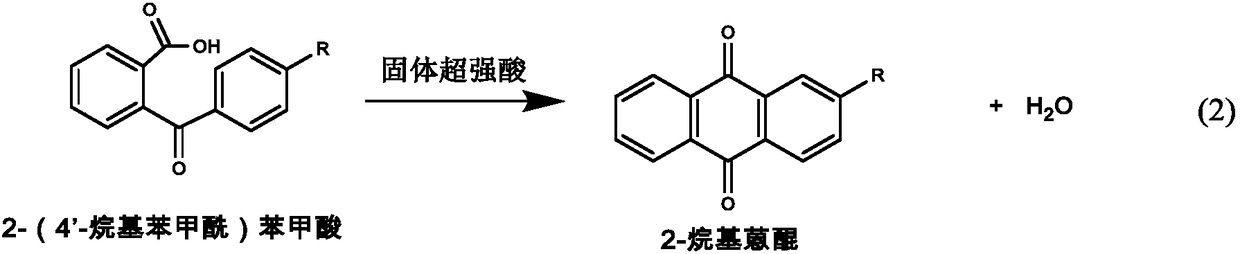
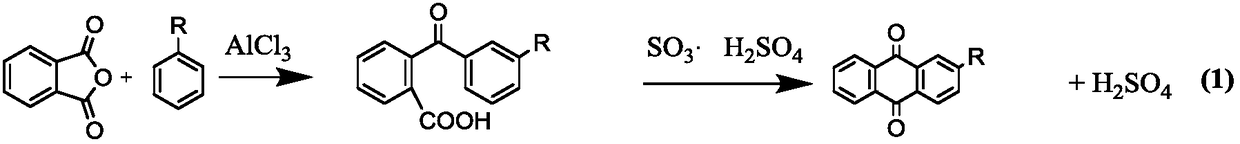Method for preparing 2-alkyl anthraquinone by taking solid super acids as catalysts
A technology of solid superacid and alkyl anthraquinone, which is applied in the direction of carboxylate preparation, carboxylic anhydride preparation, chemical instruments and methods, etc., can solve the problem of low conversion rate of 2-(benzoyl)benzoic acid and anthraquinone selectivity No high-level problems, to achieve the effect of reducing equipment maintenance and repair costs, high catalytic efficiency, and less usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 5.0g of 2-(4'-ethylbenzoyl)benzoic acid into the reactor, liquefy at 130°C, add 1.0g of perfluorosulfonic acid resin, stir and heat up to 200°C, react for 1.0 hour, cool, add Chloroform completely dissolves the reaction product, filters and separates the catalyst and the reaction solution, washes the catalyst with chloroform, and recycles after drying; the reaction solution is washed with 0.1M sodium hydroxide solution, then washed with water until neutral, dried, and distilled off under reduced pressure. solvent to obtain 2-ethylanthraquinone. The composition of the solid product is analyzed by high performance liquid chromatography and the conversion rate of raw materials and catalyst selectivity are calculated. As a result, the conversion rate of 2-(4'-ethylbenzoyl) benzoic acid is 95.1%. The selection of 2-ethylanthraquinone Sex is 76.6%.
Embodiment 2
[0033] Add 5.0g of 2-(4'-ethylbenzoyl)benzoic acid into the reactor with reflux device, add 75mL of chlorobenzene to dissolve 2-(4'-ethylbenzoyl)benzoic acid, add 1.0g Perfluorosulfonic acid resin, stirred and heated to 140°C, refluxed for 3.0 hours. After cooling, filter and separate to obtain the catalyst and the reaction liquid. The catalyst was washed with chloroform and dried in vacuum before reuse. The reaction solution was washed with 0.1M sodium hydroxide solution, and then washed with water until neutral, dried with a desiccant, and evaporated under reduced pressure to remove the solvent to obtain 2-ethylanthraquinone. The composition of the solid product was analyzed by high performance liquid chromatography and the conversion of raw material and catalyst selectivity were calculated. The conversion rate of 2-(4'-ethylbenzoyl)benzoic acid was 98.2%, and the selectivity of 2-ethylanthraquinone was 73.5%.
Embodiment 3
[0035] Add 5.0g of 2-(4'-ethylbenzoyl)benzoic acid into the reactor, liquefy at 130°C, add 2.0g of phosphotungstic acid, stir and raise the temperature to 180°C, and react for 1.0 hour. After cooling, add chloroform to dissolve the reaction product completely, filter and separate to obtain the catalyst and the reaction solution, the catalyst is washed with chloroform, and the catalyst is recovered after vacuum drying, the reaction solution is washed with 0.1M sodium hydroxide solution, then washed with water until neutral, and dried The solvent was dried, and the solvent was distilled off under reduced pressure to obtain 2-ethylanthraquinone. The composition of the solid product was analyzed by high performance liquid chromatography and the conversion of raw material and catalyst selectivity were calculated. The conversion rate of 2-(4'-ethylbenzoyl)benzoic acid was 85.5%, and the selectivity of 2-ethylanthraquinone was 58.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com