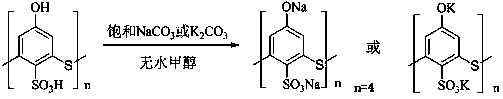Patents
Literature
196results about "Preparation from carboxylic acid anhydrides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
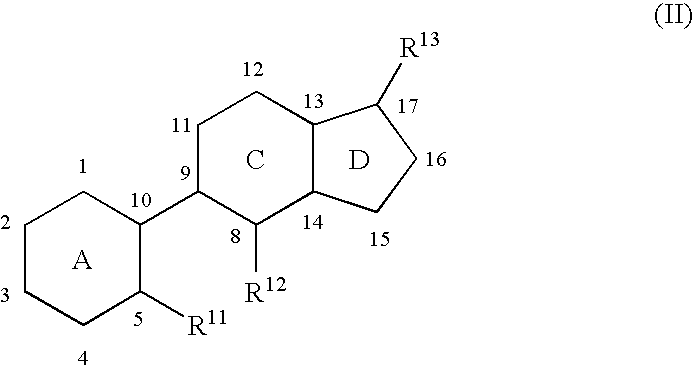
Indene derivatives as pharmaceutical agents
Owner:AQUINOX PHARMA CANADA
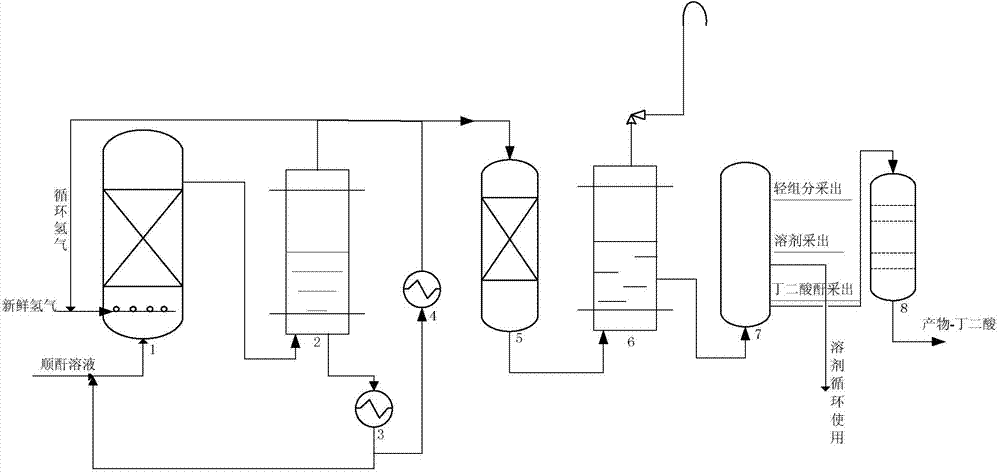
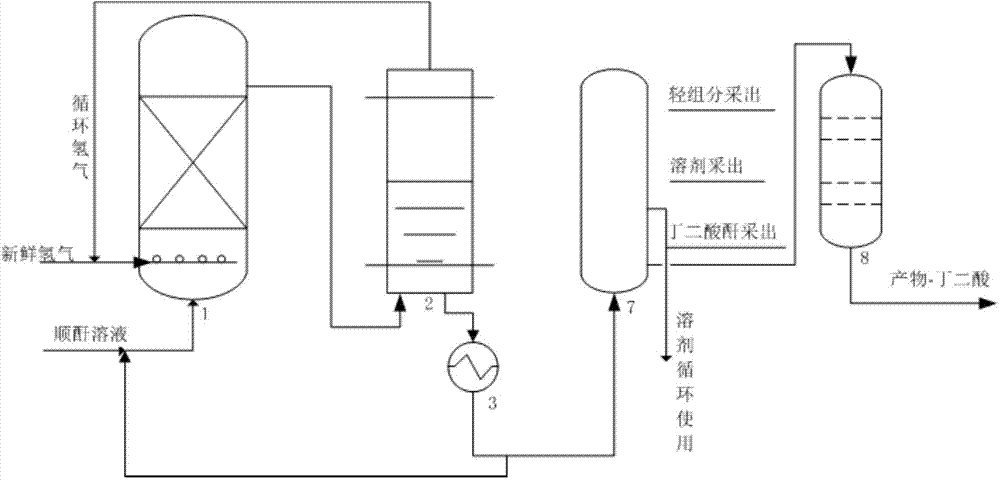
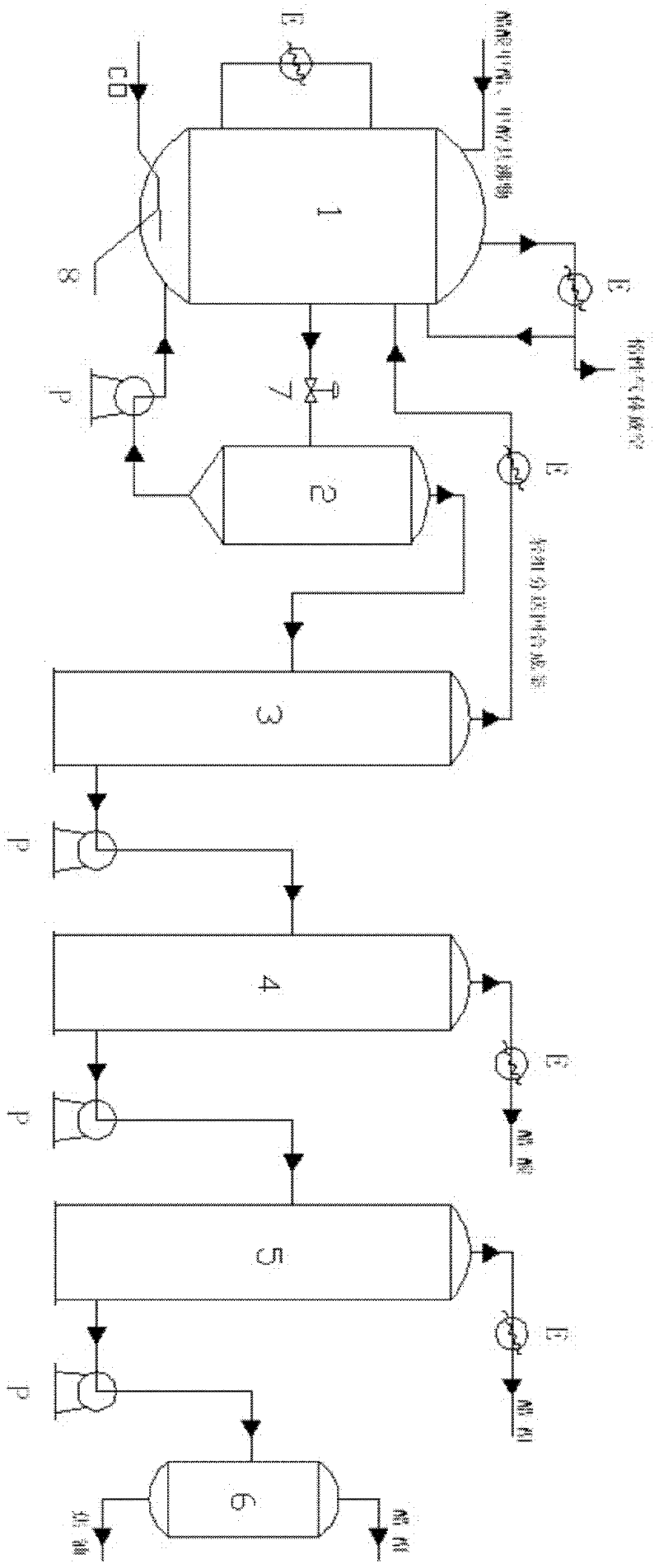
Technological process for continuously producing succinic anhydride and co-producing succinic acid through maleic anhydride hydrogenation
ActiveCN103570650AControl the average operating temperatureUniform reaction temperaturePreparation from carboxylic acid anhydridesFixed bedReaction temperature
The invention discloses a technological process for continuously producing succinic anhydride and co-producing succinic acid through maleic anhydride catalytic hydrogenation. The whole process comprises three steps, namely, reaction, rectification and hydrolysis, wherein two stages of hydrogenation reactors are used for reaction, a primary hydrogenation reactor is a fixed bed reactor with hydrogen entering from the lower part and reaction liquid exiting from the upper part, and a secondary hydrogenation reactor is a trickle bed reactor with hydrogen and reaction liquid entering from the upper part and exiting from the lower part. The technological process adopts an external circulating heat radiation manner, and reaction heat is uniformly removed, so that the average operation temperature of the whole reactor is effectively controlled, and the reaction temperature in the whole main reactor is balanced. Furthermore, the primary hydrogenation reactor adopts the manner that both the maleic anhydride solution and hydrogen flow upward simultaneously, so that the reaction temperature of the whole reactor is controlled to be balanced, local hot spot temperature is effectively controlled and lowered, and the reactants are prevented from polymerizing and depositing carbon or coking.
Owner:SHANXI UNIV
Method for producing succinic acid
ActiveCN102311332AHigh purityHigh selectivityPreparation from carboxylic acid anhydridesButanedioic acidHydrogenation reaction
The invention discloses a method for producing succinic acid. The method comprises the following steps of: performing hydrogenation reaction by taking 5 to 90 weight percent of a maleic anhydride gamma-butyrolactone solution as a raw material at the reaction temperature of between 50 and 100 DEG C under the pressure of between 0.2 and 2 MPa in the presence of a hydrogenation catalyst, hydrolyzing a hydrogenation product, crystallizing and separating to obtain succinic acid. The method is can be operated flexibly; reaction conditions are mild and controllable; and products are easy to separate and have good quality.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for degrading and recycling unsaturated polyester resin material
ActiveCN104326907AImprove immersionEfficient fractureOrganic compound preparationPreparation from carboxylic acid anhydridesFiberPolyester
The invention provides a method for degrading and recycling an unsaturated polyester resin material. The method comprises the following steps: preparing a reaction solution by a catalyst and a reaction solvent; mixing the reaction solution with an unsaturated polyester material to prepare an unsaturated polyester degrading system; heating the prepared unsaturated polyester degrading system for degradation; adding a separation solvent into the cooled unsaturated polyester degrading system; filtering to obtain solids, namely enhanced fibers and a catalyst; drying and sieving, and recycling; adding water into filtrate so as to separate out a polymer degrading component containing a styrene structure, and filtering; drying and recycling the filtered polymer solids containing the styrene structure; and evaporating filtrate to obtain substances mainly comprising resin degraded products which do not contain the styrene structure. The method for degrading and recycling the unsaturated polyester resin material has the advantages of low cost, moderate recycling conditions and high degrading activity.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI +1
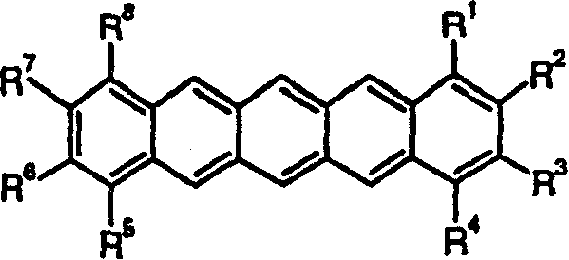
Substituted pentacene semiconductors
Substituted pentacene compounds comprise at least one substituent selected from the group consisting of electron-donating substituents, halogen substituents, and combinations thereof; the substituent(s) each being bonded to a carbon atom of a terminal ring of pentacene, and being the only substituent(s); with the proviso that when the compound has only two substituents, both of which are methyl or alkoxy, and one substituent is bonded to the number 2 carbon atom, the other substituent, if methyl, is bonded to the number 1, 3, 4, 8, or 11 carbon atom and, if alkoxy, is bonded to the number 1, 3, 4, 8, 9, or 11 carbon atom; and with the further proviso that when the compound has only four substituents, all of which are alkoxy, the substituents are bonded to the numbers 2, 3, 9, and 10 carbon atoms.
Owner:3M INNOVATIVE PROPERTIES CO
Method for preparing butanedioic acid under catalytic hydrogenation
ActiveCN101844976AContinuous productionHigh strengthPreparation from carboxylic acid anhydridesCarboxylic preparation by ozone oxidationButanedioic acidPtru catalyst
The invention discloses a method for preparing butanedioic acid under catalytic hydrogenation, which is characterized in that one or more of maleic anhydride water solution, fumaric acid water solution or maleic acid water solution are arranged inside a fixed bed reactor with catalyst to be hydrogenated to obtain butanedioic acid. By adopting the fixed bed, the catalytic hydrogenation preparationof butanedioic acid realizes the continuous mass production; transformation rate of raw materials and selectivity of the butanedioic acid reach more than 99 percent, and the purity of the product reaches more than 99.5 percent; the catalyst is of a particle-shaped load-type catalyst, and the separation of the product and the catalyst is not required; by adopting the mother liquid cycling utilization technology, raw material is saved, cost is reduced, yield can reach 100 percent, and zero emission of the three wastes is realized; and the process flow is simple, the economies of scale are large, the yield is high, and the production cost and the energy consumption are low.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
Method for preparing butanedioic acid under catalytic hydrogenation
ActiveCN101844976BContinuous productionHigh strengthPreparation from carboxylic acid anhydridesCarboxylic preparation by ozone oxidationButanedioic acidPtru catalyst
The invention discloses a method for preparing butanedioic acid under catalytic hydrogenation, which is characterized in that one or more of maleic anhydride water solution, fumaric acid water solution or maleic acid water solution are arranged inside a fixed bed reactor with catalyst to be hydrogenated to obtain butanedioic acid. By adopting the fixed bed, the catalytic hydrogenation preparationof butanedioic acid realizes the continuous mass production; transformation rate of raw materials and selectivity of the butanedioic acid reach more than 99 percent, and the purity of the product reaches more than 99.5 percent; the catalyst is of a particle-shaped load-type catalyst, and the separation of the product and the catalyst is not required; by adopting the mother liquid cycling utilization technology, raw material is saved, cost is reduced, yield can reach 100 percent, and zero emission of the three wastes is realized; and the process flow is simple, the economies of scale are large, the yield is high, and the production cost and the energy consumption are low.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
Green production method of butanedioic acid
InactiveCN107473954AImprove continuityIncrease production capacityMolecular sieve catalystsPreparation from carboxylic acid anhydridesHydration reactionButanedioic acid
The invention provides a green production method of butanedioic acid, and particularly relates to a method for producing butanedioic acid through performing catalytic hydrogenation reaction on hydrogen gas and one or free combination of several kinds of materials from maleic anhydride water solution, maleic acid water solution and fumaric acid water solution in a fixed bed tube reactor containing catalysts. According to the method, ordered mesoporous materials are used as carriers for loading precious metal to be used as hydrogenation catalysts; the generation of DL-malic acid through hydration reaction can be effectively avoided, so that the catalytic hydrogenation selectivity reaches 100 percent; the raw materials are completely converted into the butanedioic acid; the product purity reaches 99.5 percent or higher; meanwhile, the activity of the catalyst is high; the catalyst can be repeatedly used for many times. After the cooling crystallization of the water solution subjected to hydrogenation, the centrifugal mother liquid can be returned to be continuously used for preparing the hydrogenation raw materials; no waste gas, waste water and solid waste are discharged; the scaled continuous production of the butanedioic acid can be realized through the catalytic hydrogenation reaction by using the fixed bed tube reactor.
Owner:南京雪郎化工科技有限公司
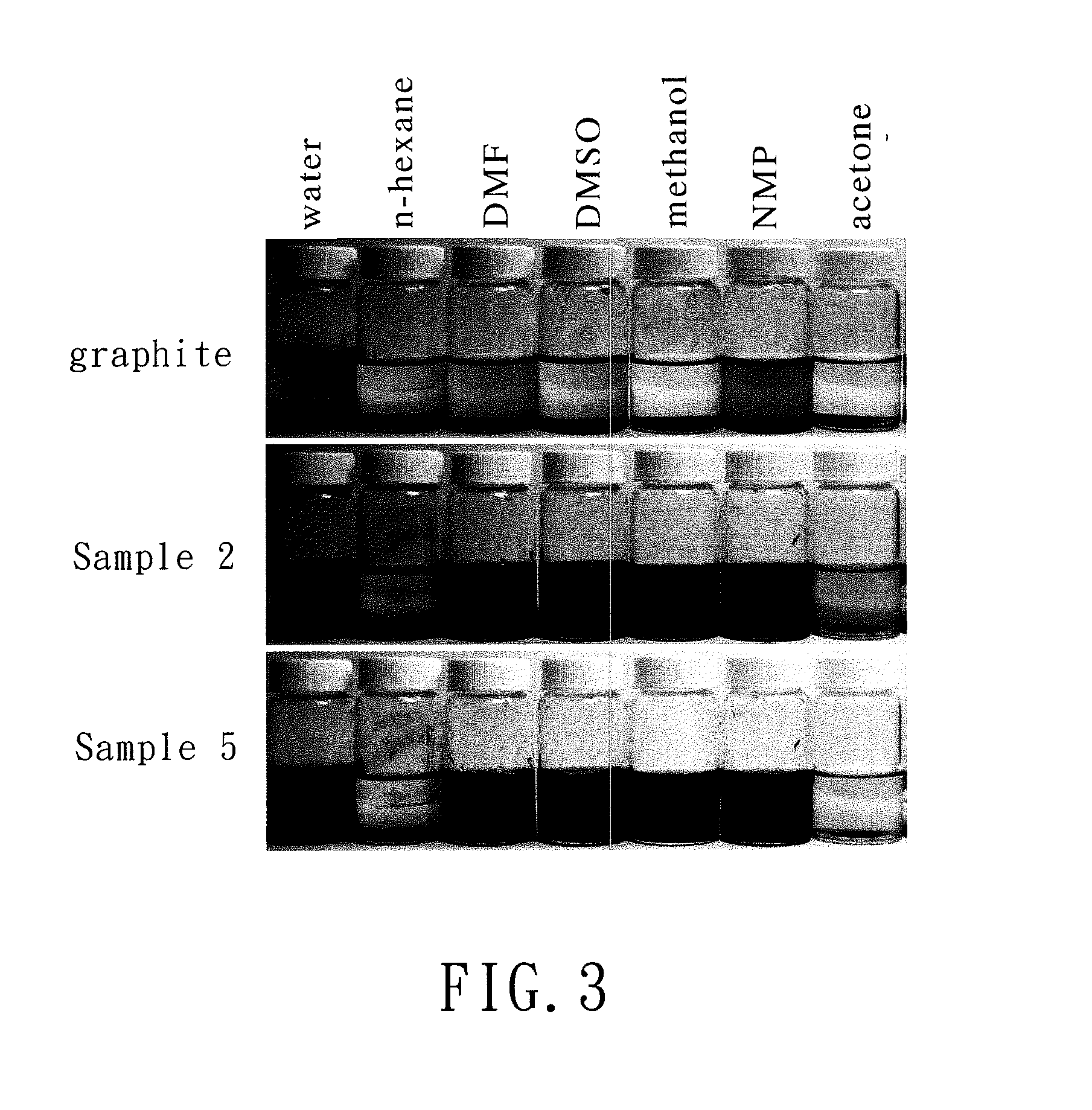
Chemically-modified graphene and method for producing the same
A chemically-modified graphene includes a graphene layer and a plurality of functional groups that are grafted to the graphene layer and each of which is represented by —CO—R—COOH, wherein R is an optionally substituted C1-C5 alkylene group or an optionally substituted C1-C5 alkenylene group. A method for producing a chemically-modified grapheme includes subjecting a cyclic anhydride and graphite to a Friedel-Crafts reaction in the presence of a Lewis acid.
Owner:CHANG GUNG UNIVERSITY
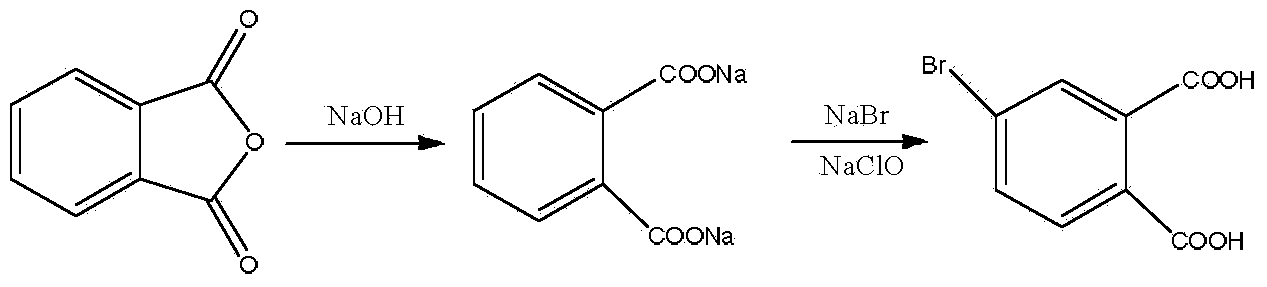
Preparation method of 4-bromophthalic acid
ActiveCN103980113AMeet the requirements of green environmental protectionReduce usageOrganic compound preparationPreparation from carboxylic acid anhydridesBrominePhthalic anhydride
The invention discloses a preparation method of 4-bromophthalic acid. The preparation method comprises the following steps: by taking phthalic anhydride, sodium hydroxide and sodium bromide as raw materials, evenly mixing phthalic anhydride and sodium hydroxide, under the ultrasonic condition, adding a sodium hypochlorite water solution for reaction, and controlling the pH value of the reaction liquor, to obtain a target product 4-bromophthalic acid. The purity of the 4-bromophthalic acid obtained by adopting the method is more than 98.5%, and the yield is more than 80%, so that the method meets the green and environment-friendly demands. Compared with the prior art, the preparation method has the advantages that the use of high-toxicity and volatile high-price bromine can be avoided, the utilization rate of bromine can achieve more than 90%, the unreacted phthalic acid can be recovered, and thus the green chemical demand is met; under the ultrasonic condition, the method is good in selectivity, the reaction is slow and easy to control, the 4-bromophthalic acid is high in yield, stable in quality and suitable for industrial production.
Owner:SINOPHARM CHEM REAGENT
Process of Making Butyric Acid
InactiveUS20080009652A1Organic compound preparationPreparation from carboxylic acid anhydridesButyric acidMaleic anhydride
Processes for forming butyric acid are provided. In one process, maleic anhydride is formed by oxidizing a hydrocarbon containing gas. The maleic anhydride is then hydrogenated in the presence of a hydrogenation catalyst to form butyric acid. The selectivity of maleic anhydride to butyric acid is at least about 35 molar percent.
Owner:HUNTSMAN PETROCHEMICAL LLC
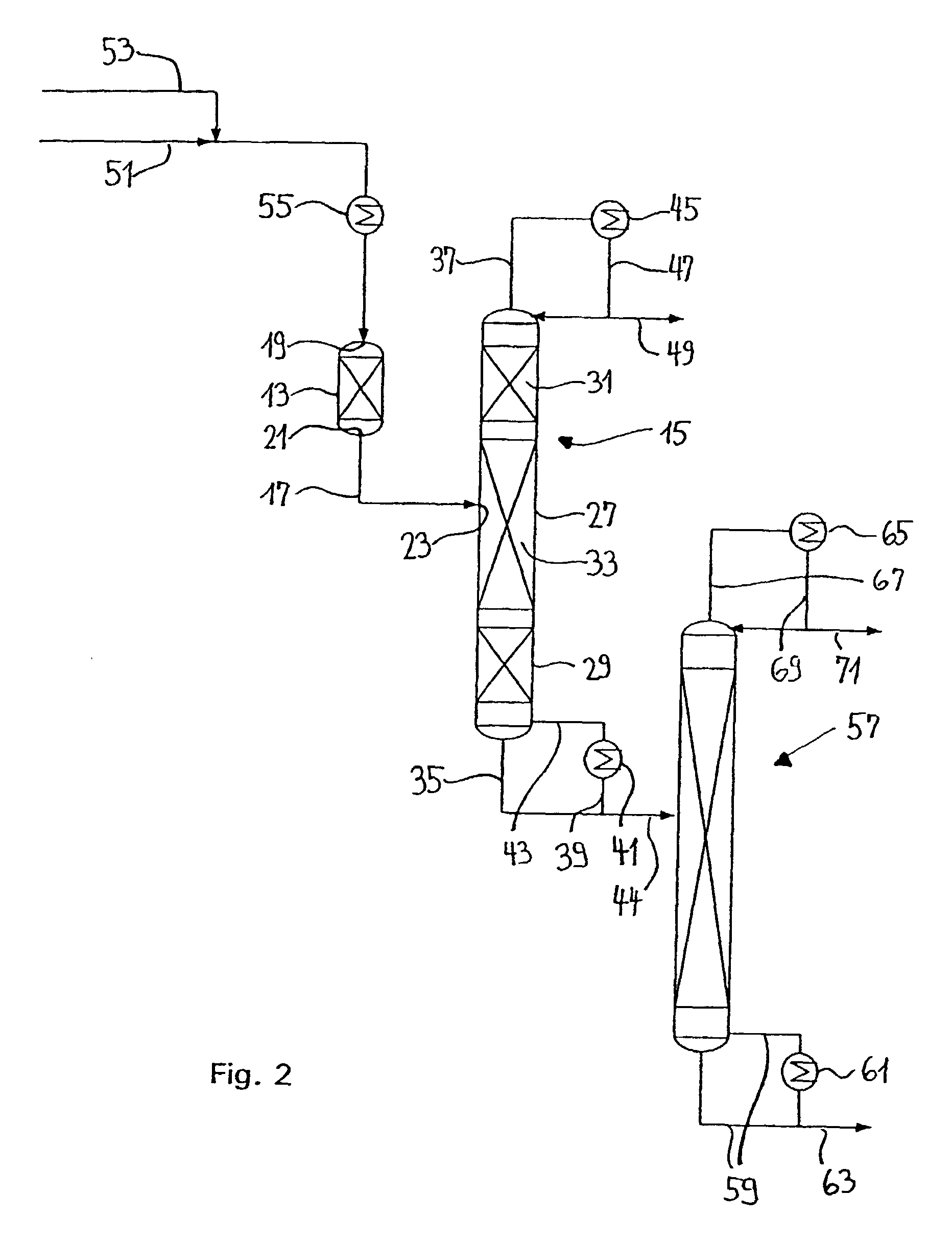
Process and device for hydrolytically obtaining a carboxylic acid alcohol from the corresponding carboxylate
InactiveUS7041199B1Raise the ratioConvenience to workSolvent extractionOrganic chemistry methodsAllyl acetateAlcohol
A process and a device for the catalytic hydrolysis of a carboxylate, e.g., methyl acetate, ethyl acetate, i- or n-propyl acetate, i- or n-butyl acetate, allyl acetate and methyl formate, into the corresponding carboxylic acid and alcohol, by a combination of a pre-reactor and a reactive distillation column are disclosed. By the combination of a pre-reactor and a reactor distillation column, the conversion ratio can be substantially improved. The device can compensate for fluctuations in the supply quantity or in the composition of the feed flow. The product composition can also be controlled in a wide range.
Owner:SULZER CHEMTECH AG +1
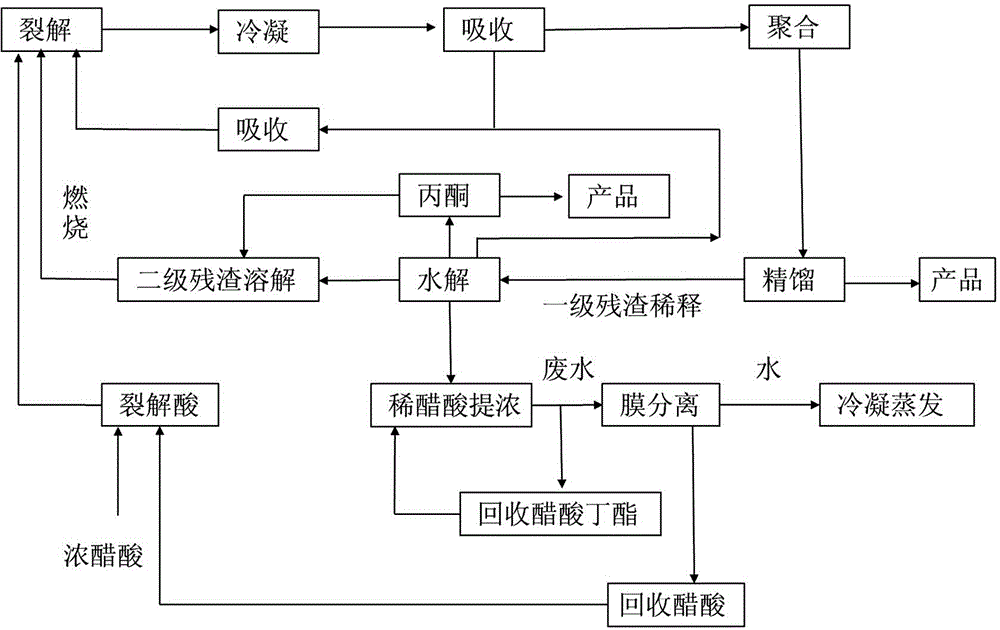
Clean production method of diketene
InactiveCN104402670ASolve the use problemOxygen-containing compound preparationOrganic compound preparationAcetic acidDistillation
The invention provides a clean production method for acetic acid cracking for preparation of diketene. The method comprises the steps of cracking, condensation, absorption, polymerization, distillation, and residue hydrolysis treatment. The method is characterized in that dilute acetic acid is added in the residue hydrolysis section to obtain acetone, acetic acid and secondary residue; the acetone is for recycling, part of the acetone is mixed with the secondary residue to provide heat for fuel production; and acetic acid is subjected to separation, extraction and concentration and enters the cracking section as a production raw material. The invention has the advantages that by optimizing the process and the circular route, the generated waste gas and residue in the production process are digested and absorbed in the system, so as to realize clean production.
Owner:ANHUI JINGHE IND
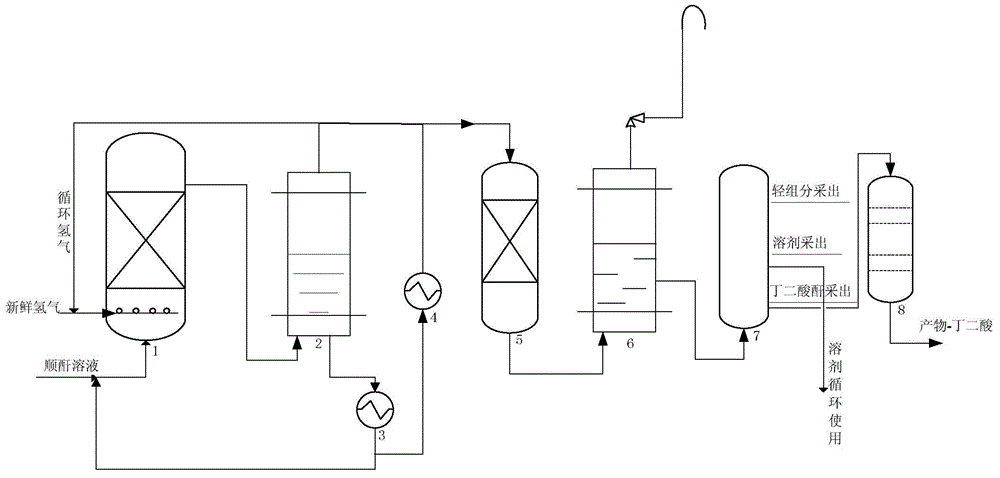
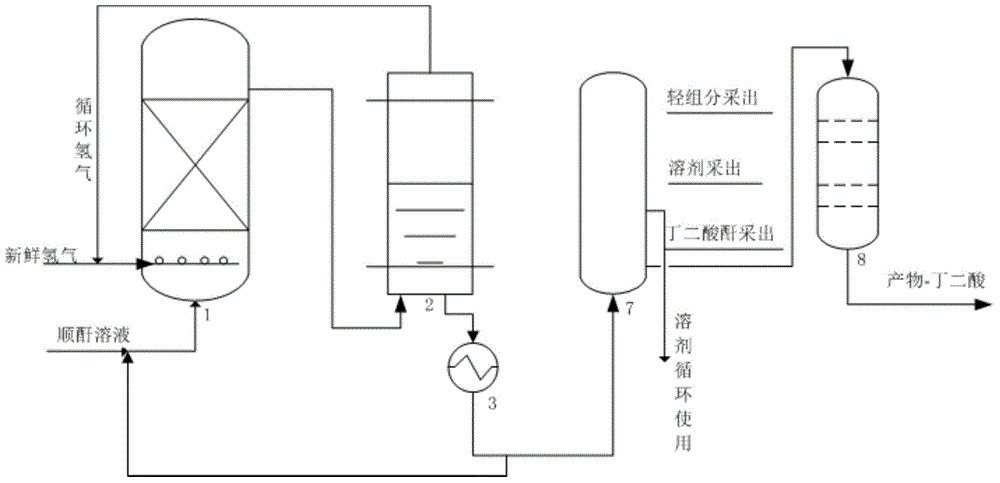
Hydrogenation catalyst, preparation method and application thereof, and method for preparing succinic acid through maleic anhydride hydrogenation
ActiveCN114433127AHigh catalytic activityImprove stabilityHeterogenous catalyst chemical elementsPreparation from carboxylic acid anhydridesPolymer sciencePtru catalyst
The invention relates to the technical field of catalysts, in particular to a hydrogenation catalyst, a preparation method and application thereof and a method for preparing succinic acid through maleic anhydride hydrogenation, the catalyst comprises a carrier and active components loaded on the carrier, the active components contain oxides of Ce, Ni and Ru, and the carrier contains SiO2; ru is distributed on the outer layer of the catalyst, and oxides of Ni and Ce are distributed in the catalyst; in the catalyst, relative to the carrier, the content of Ni is 1-15 wt%, the content of Ce oxide is 0.5-7 wt%, and the content of Ru is 0.1-2 wt%. The hydrogenation catalyst has excellent catalytic activity, stability and succinic acid selectivity, and is low in cost and simple in process. The catalyst provided by the invention can be used for preparation of succinic acid by continuous hydrogenation of maleic anhydride at a low catalytic reaction temperature (70-180 DEG C).
Owner:CHINA PETROLEUM & CHEM CORP +1
Technological process for continuously producing succinic anhydride and co-producing succinic acid through maleic anhydride hydrogenation
ActiveCN103570650BControl the average operating temperatureUniform reaction temperaturePreparation from carboxylic acid anhydridesFixed bedReaction temperature
The invention discloses a technological process for continuously producing succinic anhydride and co-producing succinic acid through maleic anhydride catalytic hydrogenation. The whole process comprises three steps, namely, reaction, rectification and hydrolysis, wherein two stages of hydrogenation reactors are used for reaction, a primary hydrogenation reactor is a fixed bed reactor with hydrogen entering from the lower part and reaction liquid exiting from the upper part, and a secondary hydrogenation reactor is a trickle bed reactor with hydrogen and reaction liquid entering from the upper part and exiting from the lower part. The technological process adopts an external circulating heat radiation manner, and reaction heat is uniformly removed, so that the average operation temperature of the whole reactor is effectively controlled, and the reaction temperature in the whole main reactor is balanced. Furthermore, the primary hydrogenation reactor adopts the manner that both the maleic anhydride solution and hydrogen flow upward simultaneously, so that the reaction temperature of the whole reactor is controlled to be balanced, local hot spot temperature is effectively controlled and lowered, and the reactants are prevented from polymerizing and depositing carbon or coking.
Owner:SHANXI UNIV
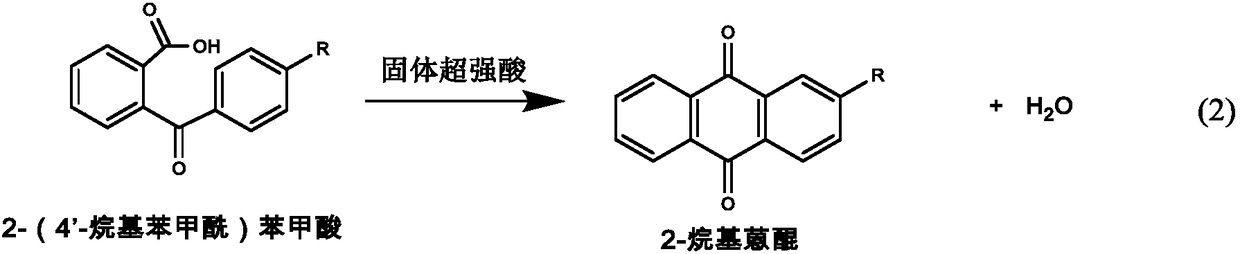
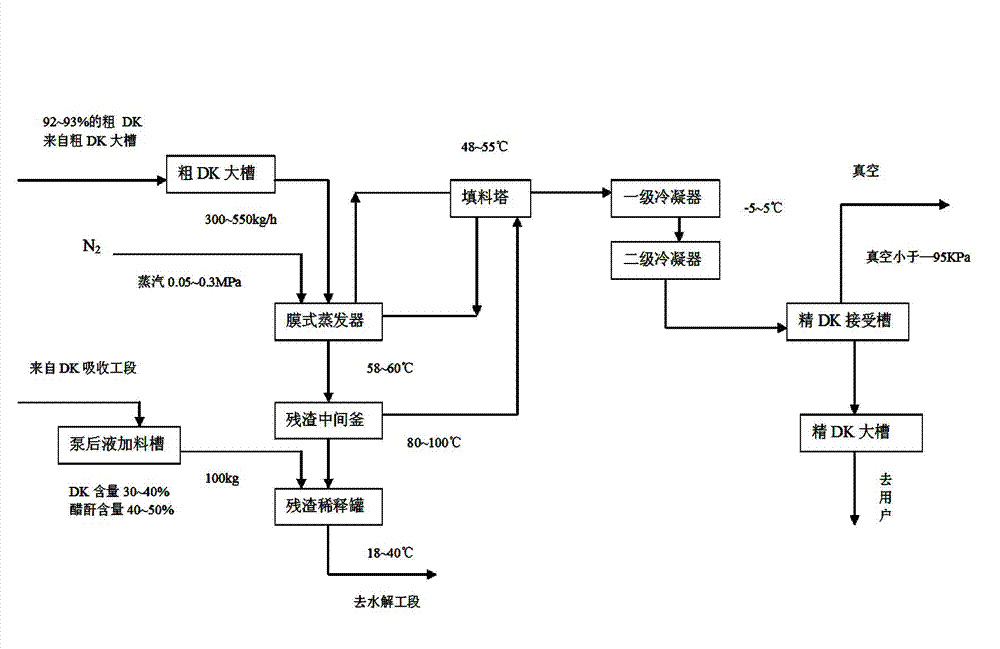
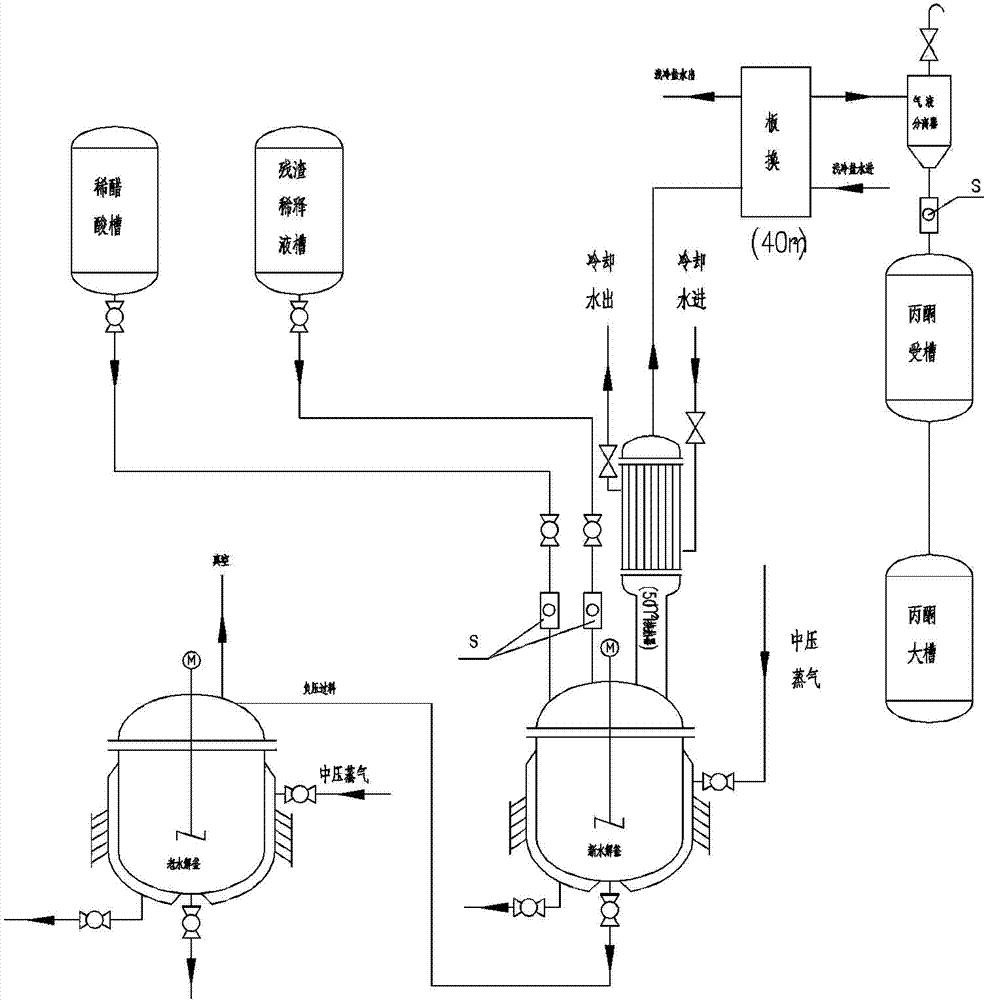
Method for preparing 2-alkyl anthraquinone by taking solid super acids as catalysts
InactiveCN108299176AHigh catalytic efficiencyReduce usageOrganic compound preparationPreparation from carboxylic acid anhydridesBenzoic acidHeteropoly acid
The invention provides a method for preparing 2-alkyl anthraquinone by taking solid super acids as catalysts. The 2-alkyl anthraquinone (alkyl is straight or branched alkyl with the number of carbon atoms of 1-6) is obtained by adopting the solid super acids like perfluorinated sulfonic acid resin and heteropoly acid as the catalysts, taking 2-(4'-alkyl benzoyl) benzoic acid as a raw material andperforming acylated dewatering closed loop through Friedel-Crafts reaction. The method provided by the invention is characterized in that traditional smoking sulfuric acid catalysts are replaced by the solid super acids, so that the environment is friendly, and no waste acid is discharged; the operation process is simple, and the solid catalysts are easy to recover; therefore, the method is a green pollution-free new process.
Owner:BEIJING UNIV OF CHEM TECH
Processes for purification of acid solutions
ActiveUS20120123160A1Long catalyst lifeEasy to usePreparation from carboxylic acid anhydridesCarboxylic compound separation/purificationCorrosion resistant alloyFractionating column
The invention provides processes for purification of streams containing carboxylic acids and carboxylic acid anhydrides without using the amount of high-cost, corrosion resistant alloy required for a distillation column. The invention provides methods in which streams containing carboxylic acids and carboxylic acid anhydrides are subjected to a hydrolysis process by combining them with a stoichiometric excess of water and optionally an added hydrolysis catalyst. The resulting hydrolyzed stream is subsequently separated to produce a stream containing carboxylic acid and water and a carboxylic acid product stream comprising carboxylic acid.
Owner:EASTMAN CHEM CO
Treating method and treating device for ketene dimer production residues
ActiveCN103113186AEasy to handleEfficient recyclingOxygen-containing compound preparationOrganic compound preparationPlate heat exchangerEthenone
Owner:ANHUI JINGHE IND
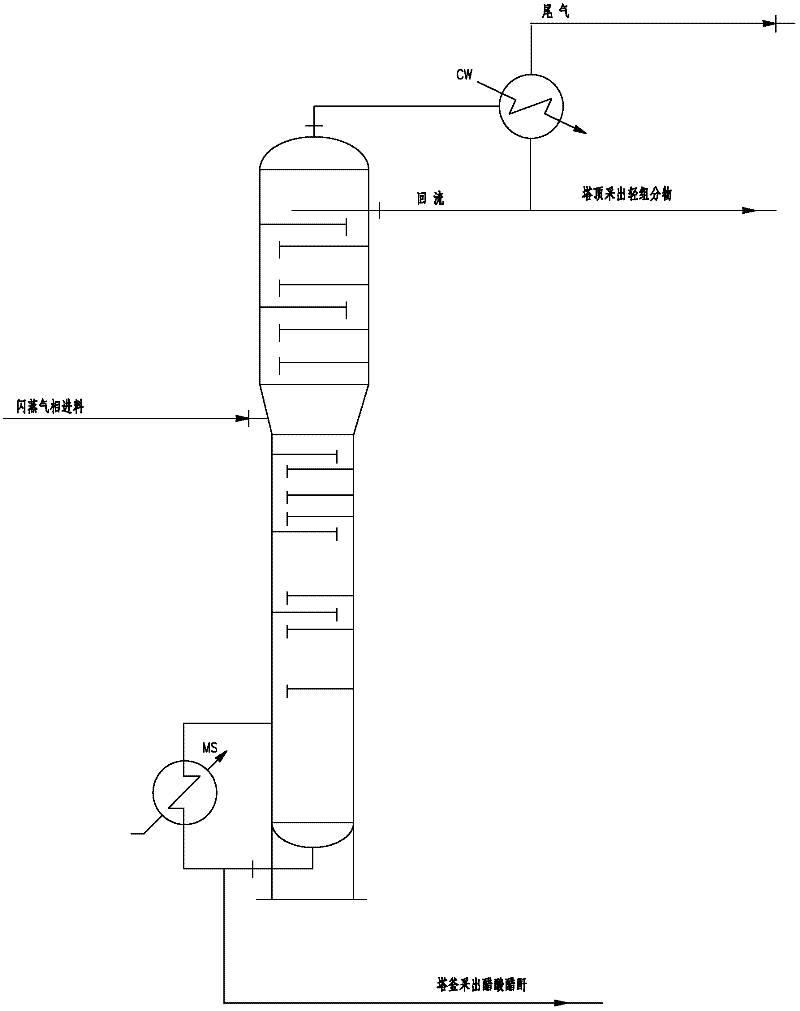
Method for preparation of acetic anhydride and acetic acid by means of carbonyl synthesis of methyl acetate and methanol azeotrope and method for separation
ActiveCN102381956ASolve the use problemEmission reductionOrganic compound preparationPreparation from carboxylic acid anhydridesAcetic anhydrideTriiodide
The invention discloses a method for preparation of acetic anhydride and acetic acid by means of carbonyl synthesis of methyl acetate and methanol azeotrope. The synthesis reaction is operated in a carbonyl synthesizing kettle (1) which is provided with a mixer, a bypass circulation device controlled by a high-pressure pump and a gas distributor (8) at the bottom. The method includes steps of adding azeotrope and catalyst from the upper portion of the kettle, and gaseous carbon monoxide is fed into the kettle from the bottom of the kettle through the gas distributor (8). The internal pressureof the kettle is 3.0-4.5MPa, and the reaction temperature is 140-220 GEG C. The catalyst includes rhodium catalyst, catalysis-supporting methyl iodide and lithium acetate, the rhodium catalyst with the concentration ranging from 200ppm to 800ppm is selected from rhodium triiodide or rhodium chloride, adding quantity of the methyl iodide is 10-30% of that of the azeotrope, and the concentration ofthe lithium acetate is two to three times of that of the rhodium catalyst. Reaction liquid sequentially passes through a flash evaporation kettle (2), a light component removing tower (3), an acetic acid tower (4) and an acetic anhydride tower (5) to obtain acetic anhydride with purity higher than 97% and acetic acid with purity higher than 99%. The method effectively resolves the problem of comprehensive utilization of methyl acetate and methanol azeotrope.
Owner:ANHUI WANWEI UPDATED HIGH TECH MATERIAL CO LTD
Device and process for continuously producing chloroacetic acid by differential circle flow
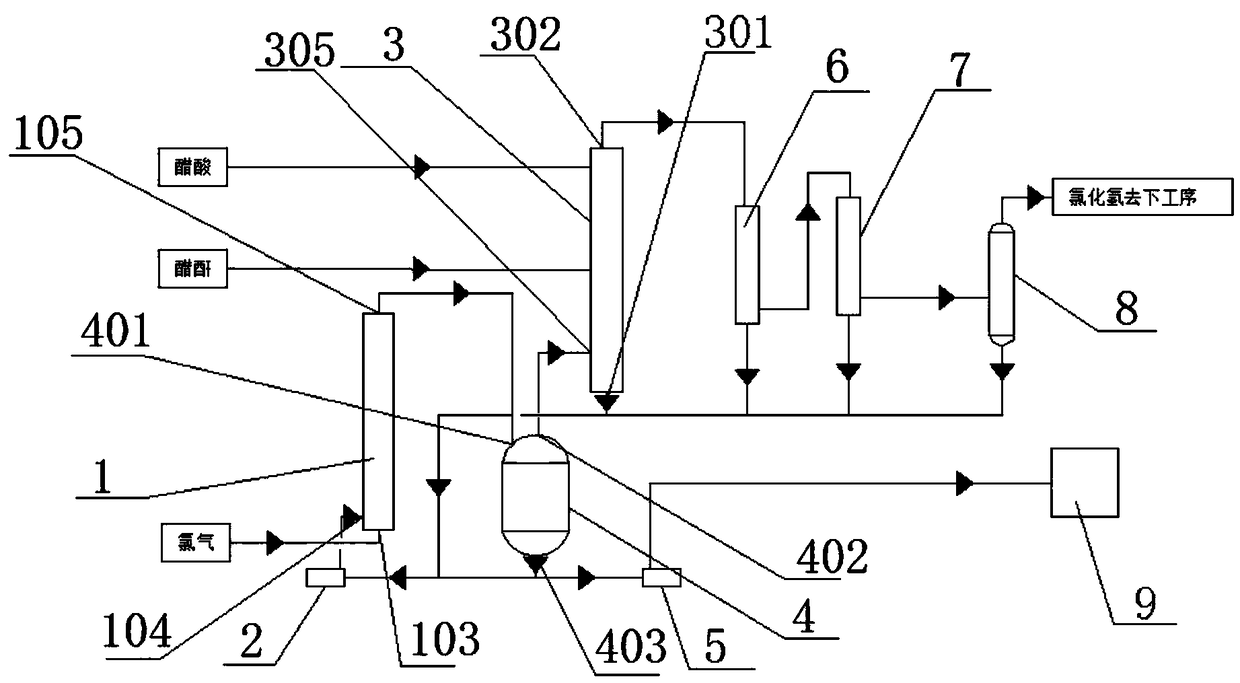
PendingCN109134231AReduce generationLess investmentChlorine/hydrogen-chloride purificationPreparation from carboxylic acid anhydridesAcetic acidAutomatic control
The invention provides a device and process for continuously producing chloroacetic acid by differential circle flow. The device comprises a main reactor, a pre-reactor, a separator, a circulating pump, a primary condenser, a secondary condenser, a demister, a discharge pump, pipelines and automatic control instruments, wherein both the main reactor and the pre-reactor are a tubular micro-channelreaction heat exchanger; a plurality of micro-channels are arranged in the main reactor; the main reactor is provided with a plurality of longitudinal main-reactor tube passes and a plurality of transverse main-reactor shell passes; the main-reactor shell passes are connected with a main-reactor cooling water inlet and a main-reactor cooling water outlet respectively arranged on two sides of the main reactor; and a gas-liquid mixing chamber is arranged at the bottom of the main reactor. Compared with the traditional technology, the device and process have the following advantages: a hydrodechlorination device and an auxiliary hydrogen chloride absorption device are saved, the equipment investment cost is reduced, generated polychlorinated compounds in the chlorination liquid are reduced bythe differential circle flow process and the production efficiency and quality of chloroacetic acid are improved.
Owner:杭州众立化工科技有限公司
Free-polyunsaturated-fatty-acid-containing composition and method for manufacturing same
ActiveUS20180187126A1Easy to handleOrganic active ingredientsFatty acid esterificationAlcoholContact time
Provided is a free-polyunsaturated-fatty-acid-containing composition that has a total metal content of 0.1 ppm or less and that comprises at least one free polyunsaturated fatty acid having 20 or more carbon atoms, in an amount that is at least 80.0% of the amount of fatty acids in the composition; and a method for manufacturing a free-polyunsaturated-fatty-acid-containing composition, comprising: providing a raw material composition containing at least one polyunsaturated fatty acid having 20 or more carbon atoms; performing a hydrolysis treatment on a reaction solution prepared by combining the provided raw material composition, a lower alcohol, water having a total metal content of 0.01 ppm or less, and an alkali catalyst; and limiting the contact between the reaction composition and the metal after the hydrolysis treatment so that the product T [cm2×days] of the contact surface area [cm2] per 1 g and the contact time [days] between the composition and the metal is 100 or less.
Owner:NIPPON SUISAN KAISHA LTD
Tert-carboxylic acid synthesis method
ActiveCN105218354ASimple processImprove economyPreparation from carboxylic acid anhydridesChemical recyclingCarboxylic acidAlkene
The invention discloses a tert-carboxylic acid synthesis method. According to the method, olefin and carbon monoxide serve as raw materials, and a tert-carboxylic acid product is obtained through catalyzed synthesis under normal pressure or low pressure in the presence of strongly acidic ionic liquid-metal carbonyl complex catalyst. Corrosion is avoided, catalyst consumption is low, three-waste discharge is low, the tert-carboxylic acid product and the catalyst can be separated easily after reaction ends, and the defects of existing technologies that separation effect is poor and dilute sulphuric acid recovery is hard are overcome.
Owner:WANHUA CHEM GRP CO LTD
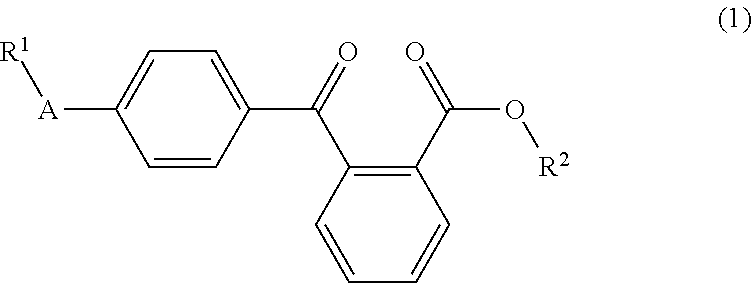
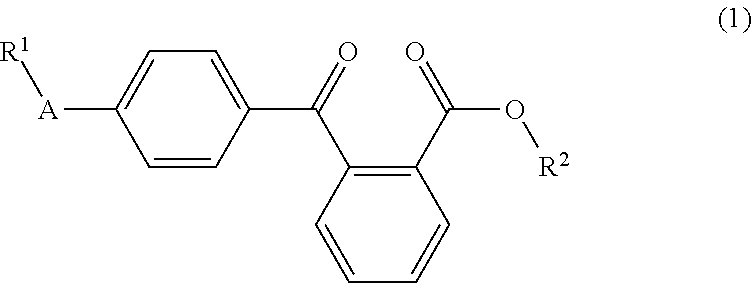
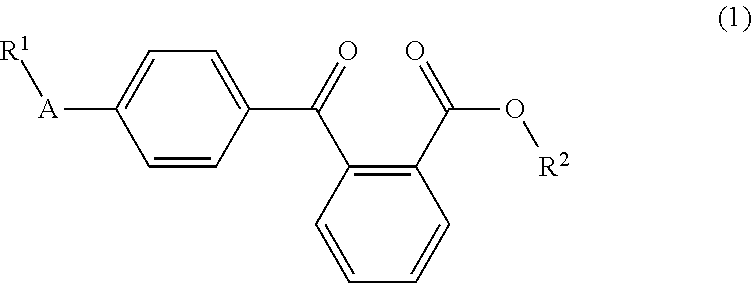
Liquid arylbenzoyl benzoic acid ester derivatives for energy curable compositions
4-Arylbenzoyl benzoic acid esters, such as the ethylhexyl esters and amyl esters of 4-phenylbenzoyl benzoic acid, are described, which are liquid and show excellent solubility in acrylic or methacrylic monomers. Compounds of Formula 1, wherein R1, R2, and A are as defined herein, are disclosed. They are suitable as components for radical photoinitiator systems for UV-curable compositions.
Owner:SUN CHEM CORP
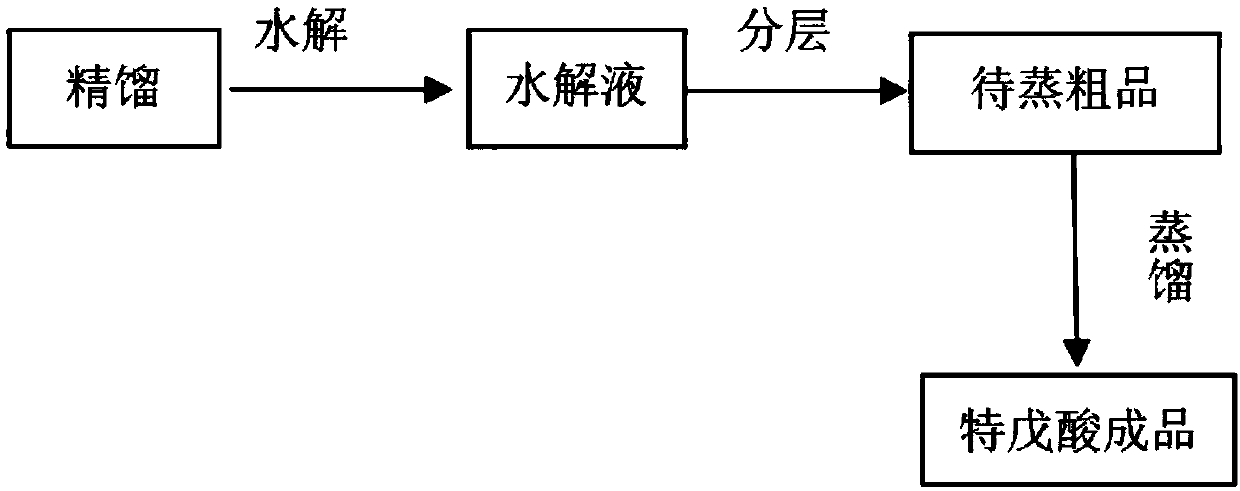
Method for recycling trimethylacetic acid from pivaloyl chloride rectification raffinate
PendingCN107721841AIncrease profitReduce pressure on environmental protectionPreparation from carboxylic acid halidePreparation from carboxylic acid anhydridesPhosphorous acidRaffinate
The invention provides a method for recycling trimethylacetic acid from pivaloyl chloride rectification raffinate. According to the technical scheme, the method has the experiment ideas that components and contents of pivaloyl chloride rectification raffinate are analyzed firstly on the basis of experiment means, on the basis, procedures such as hydrolysis, layering and rectification are confirmedaccording to chemical properties of pivaloyl chloride and trimethylacetic anhydride, and trimethylacetic acid is recycled. Specifically, the method comprises the following steps: firstly, adding a certain amount of pivaloyl chloride rectification raffinate, controlling specific temperature and pressure, adding a proper amount of water for hydrolysis reactions for multiple times to sequentially convert pivaloyl chloride and trimethylacetic anhydride into trimethylacetic acid, leaving to stand to layer water-containing phosphorous acid and a crude trimethylacetic acid product, feeding back thecrude trimethylacetic acid product to a trimethylacetic acid rectification procedure, and performing decoloring refining. By adopting the method provided by the invention, more than 90% of the pivaloyl chloride rectification raffinate can be converted into trimethylacetic acid, so that the utilization rates of raw materials are increased, the wastes are reduced, the environment protection burden of companies is alleviated, and meanwhile the production cost of products is effectively reduced.
Owner:山东民基新材料科技有限公司
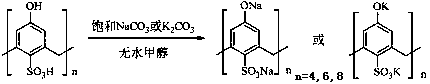
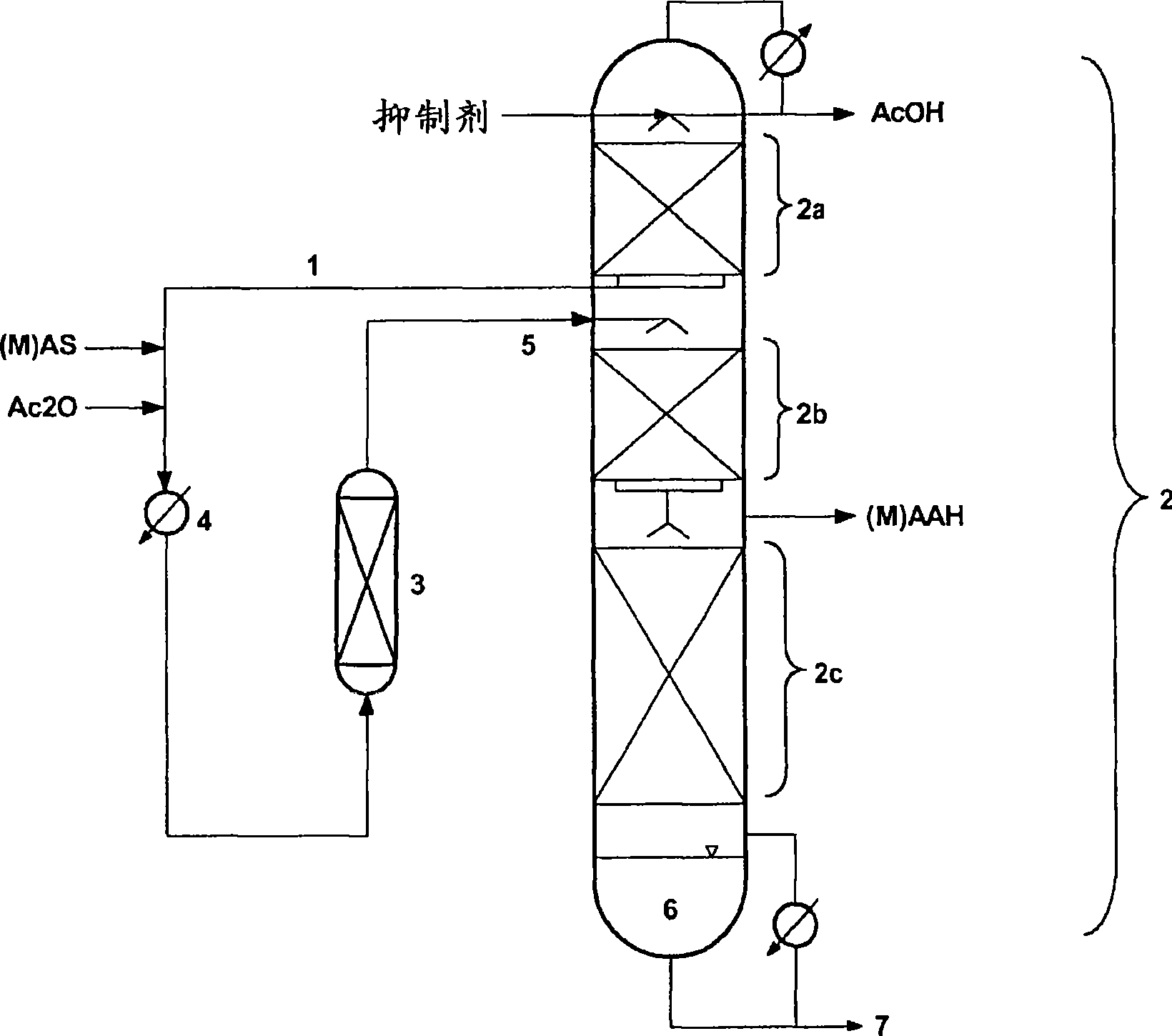
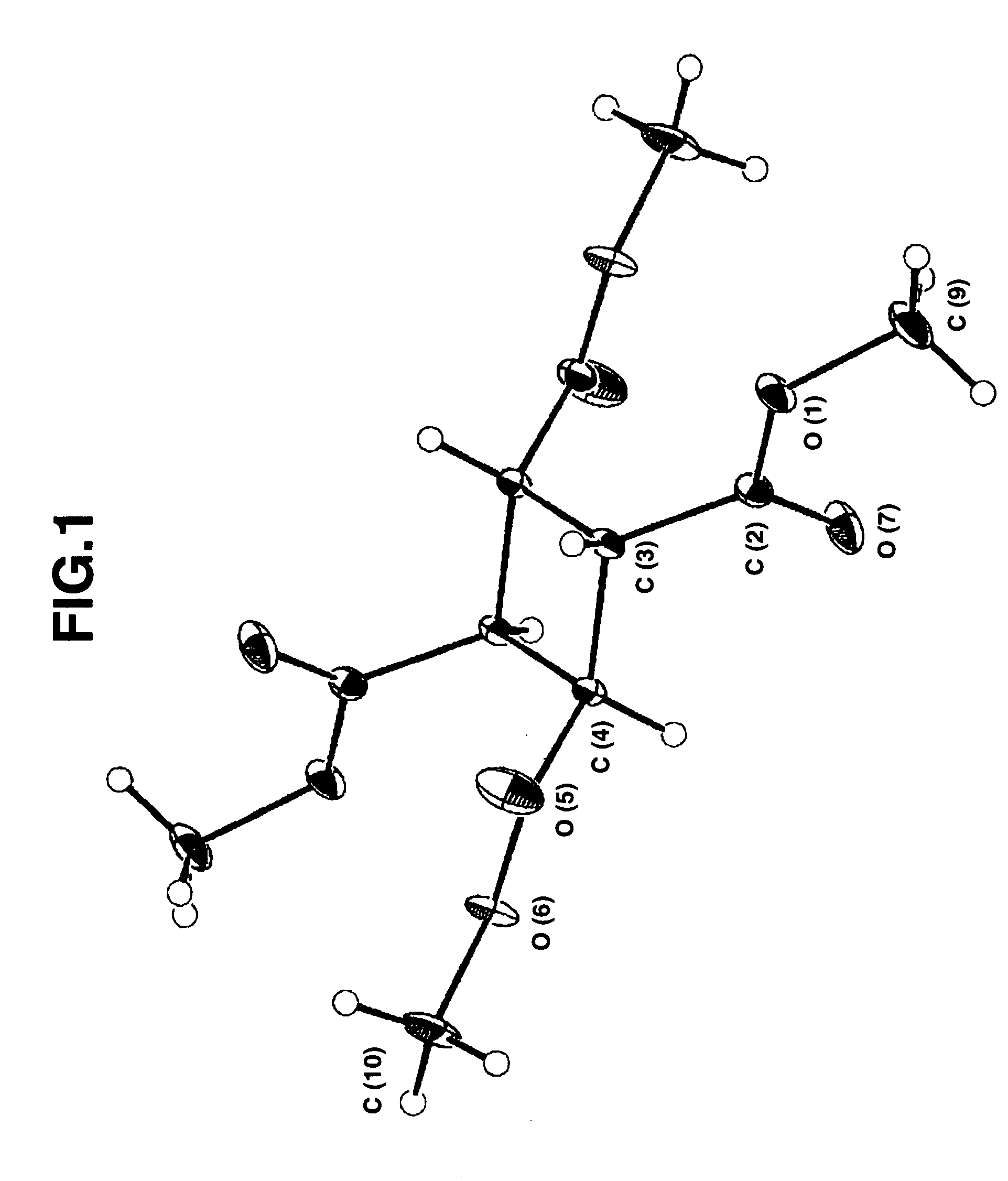
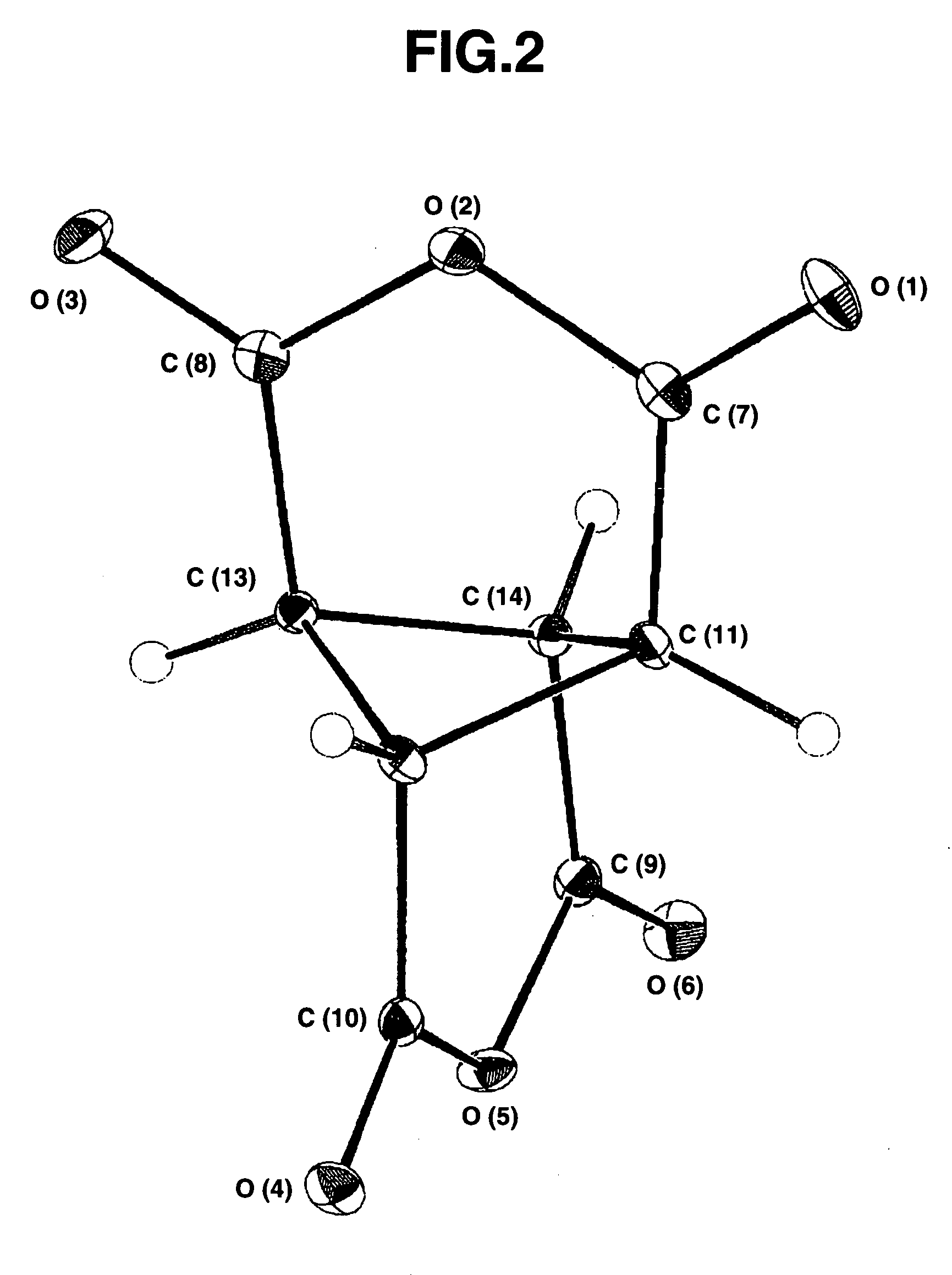
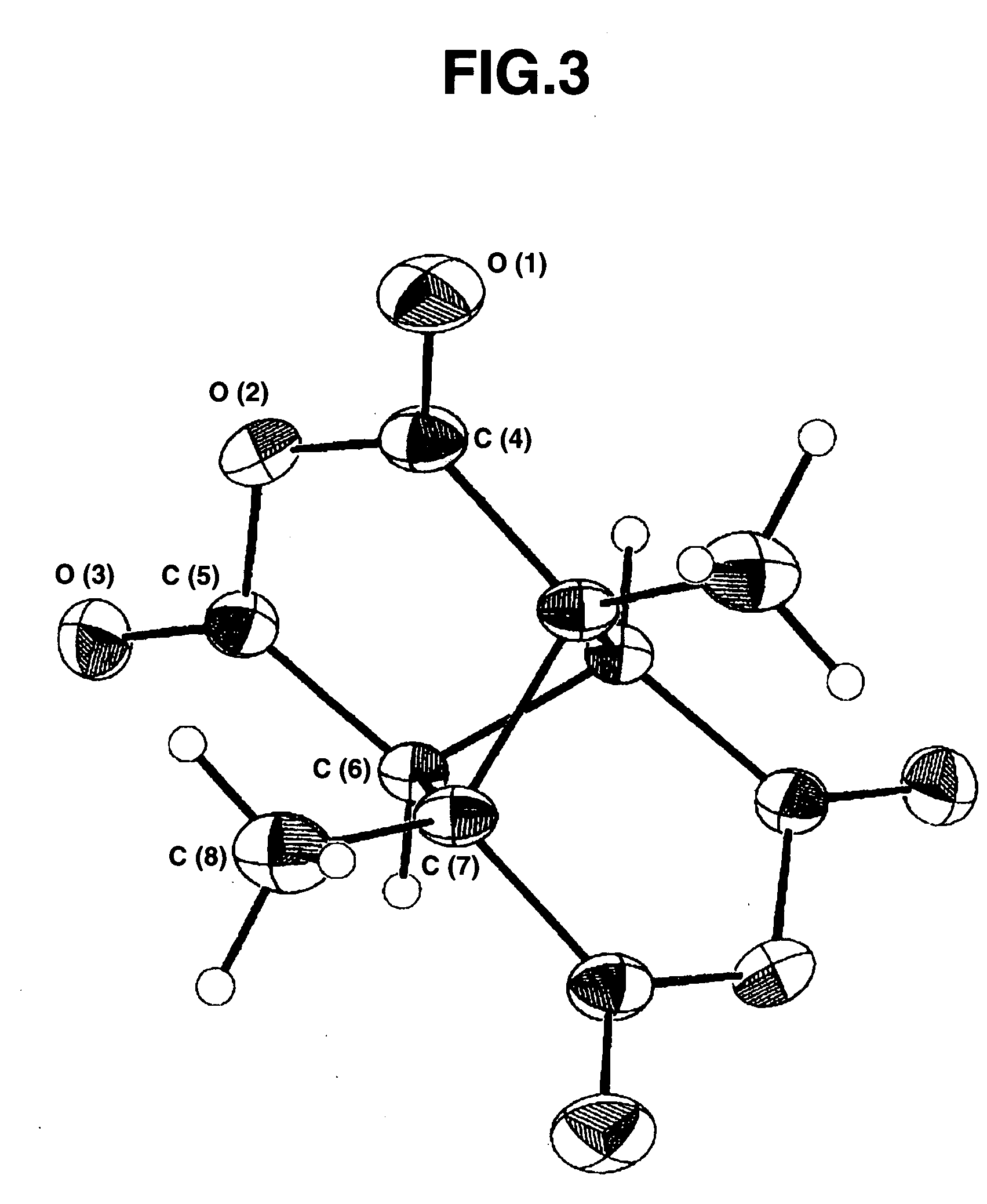
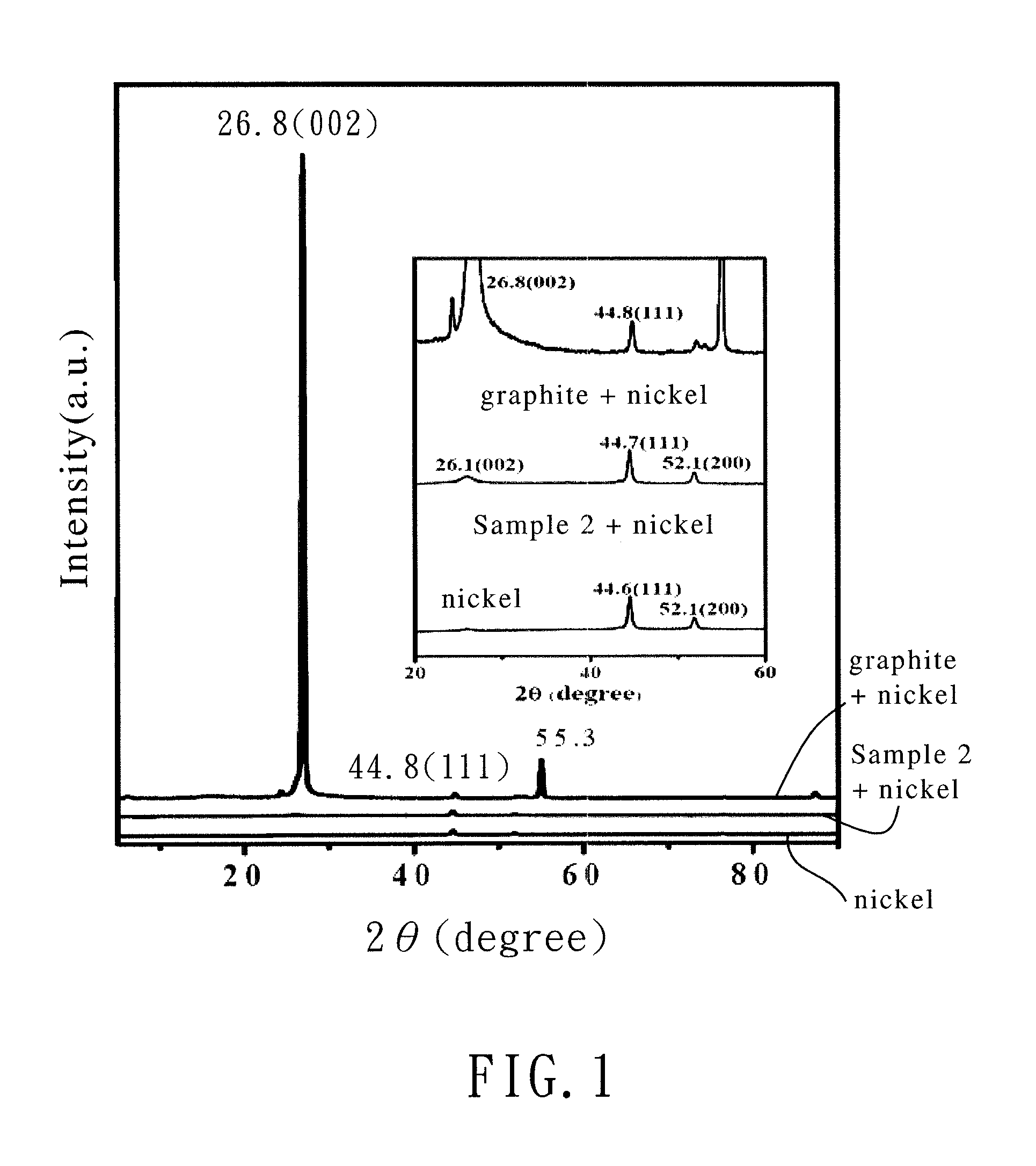
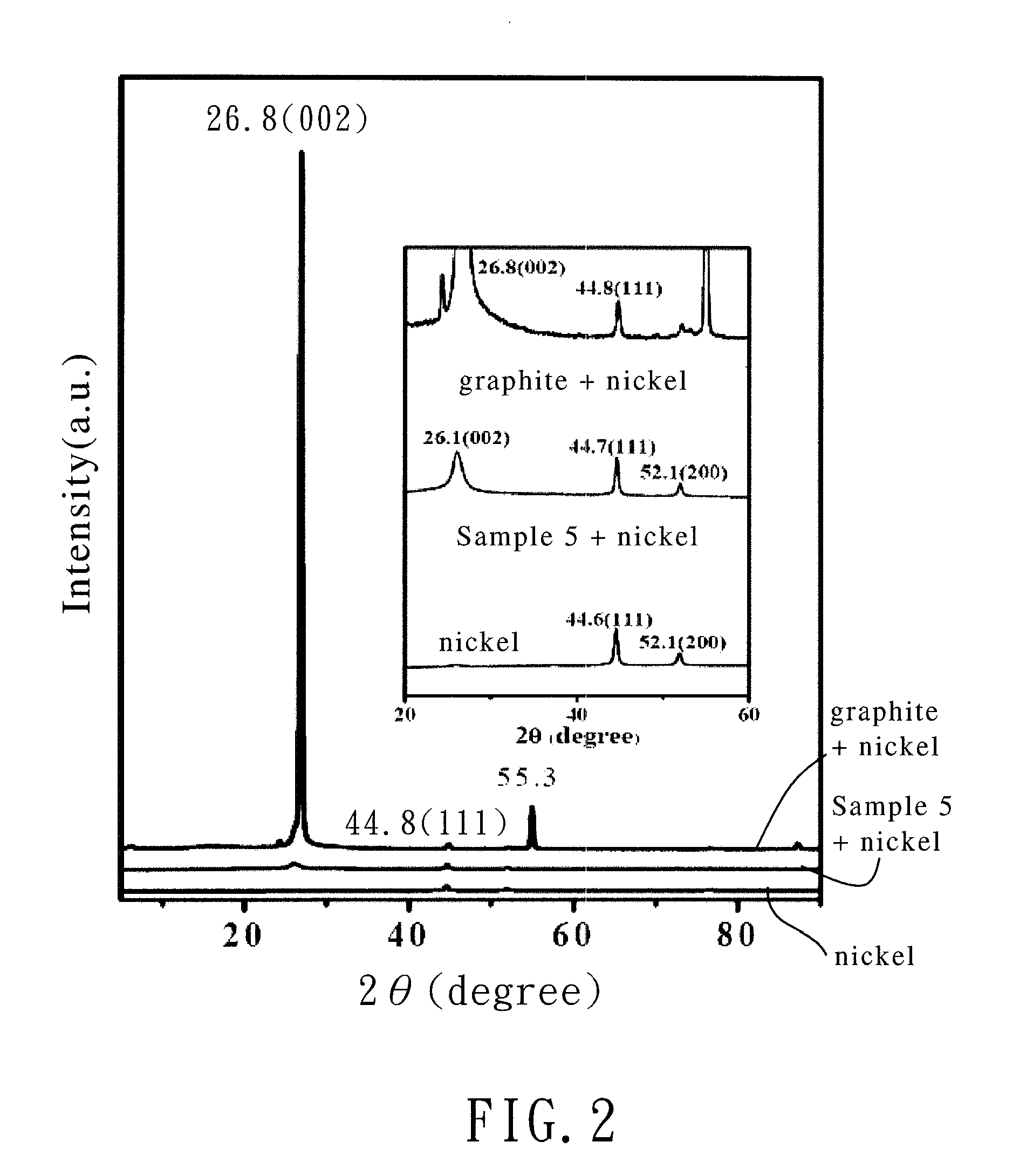
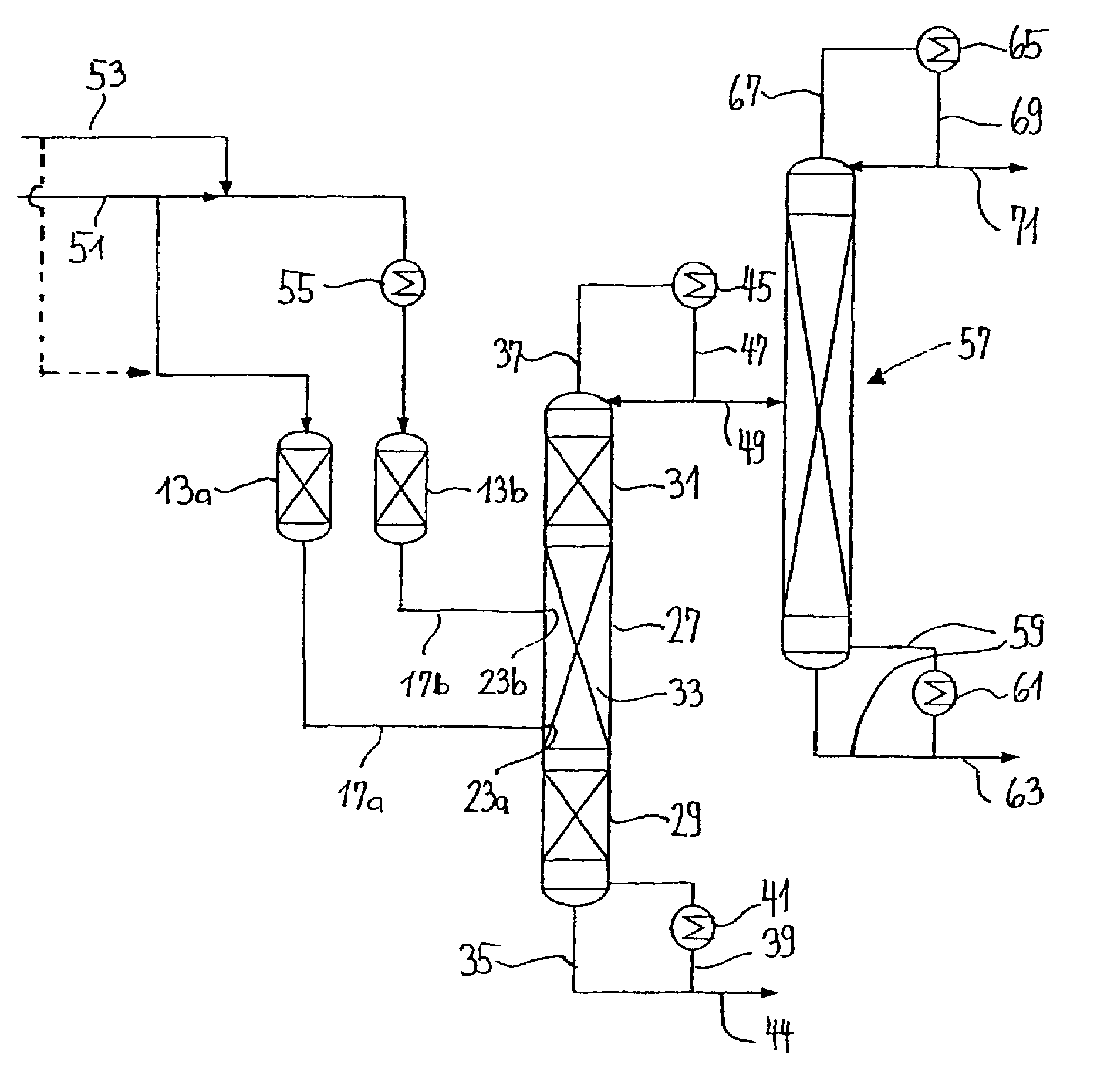
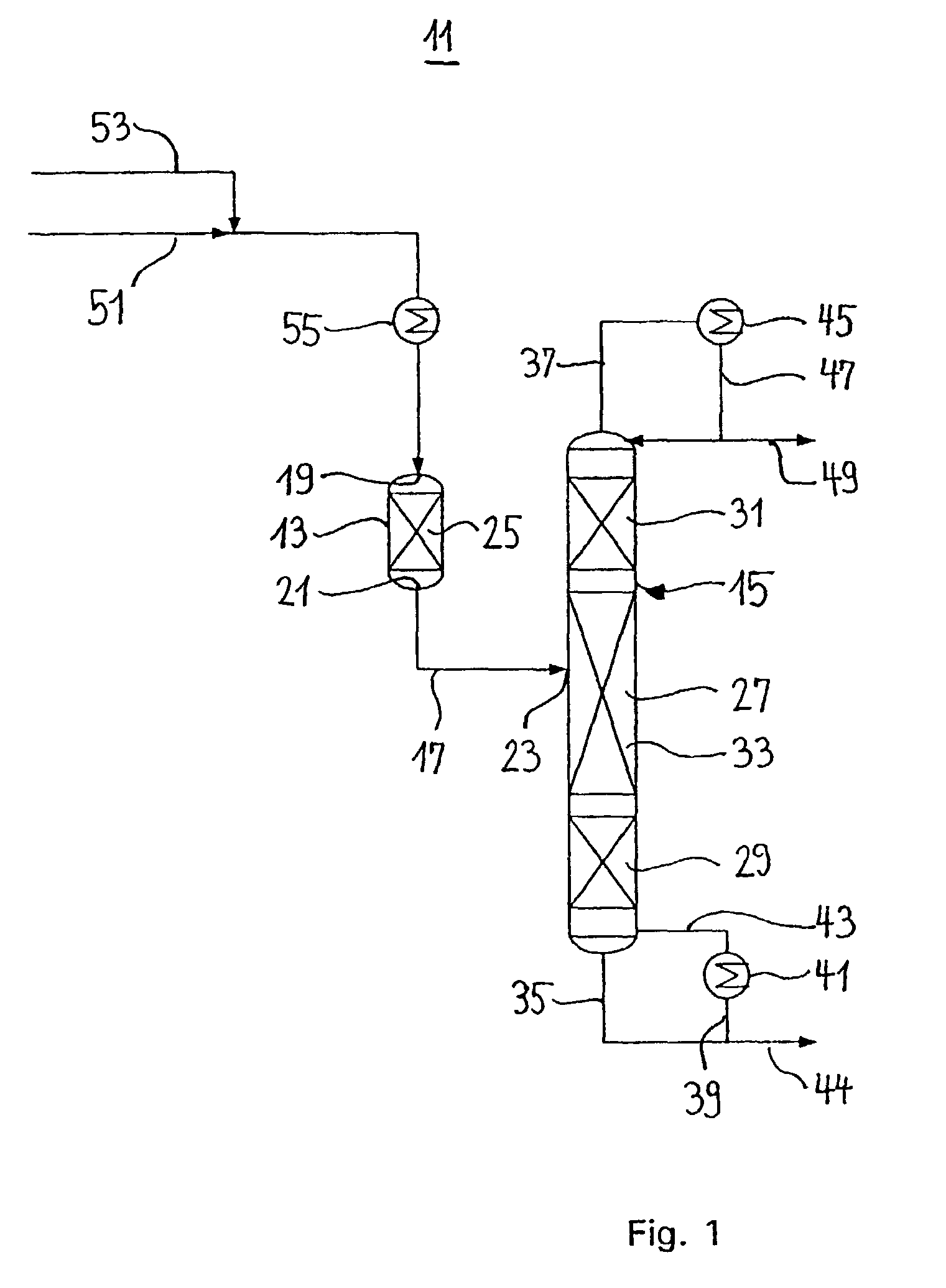
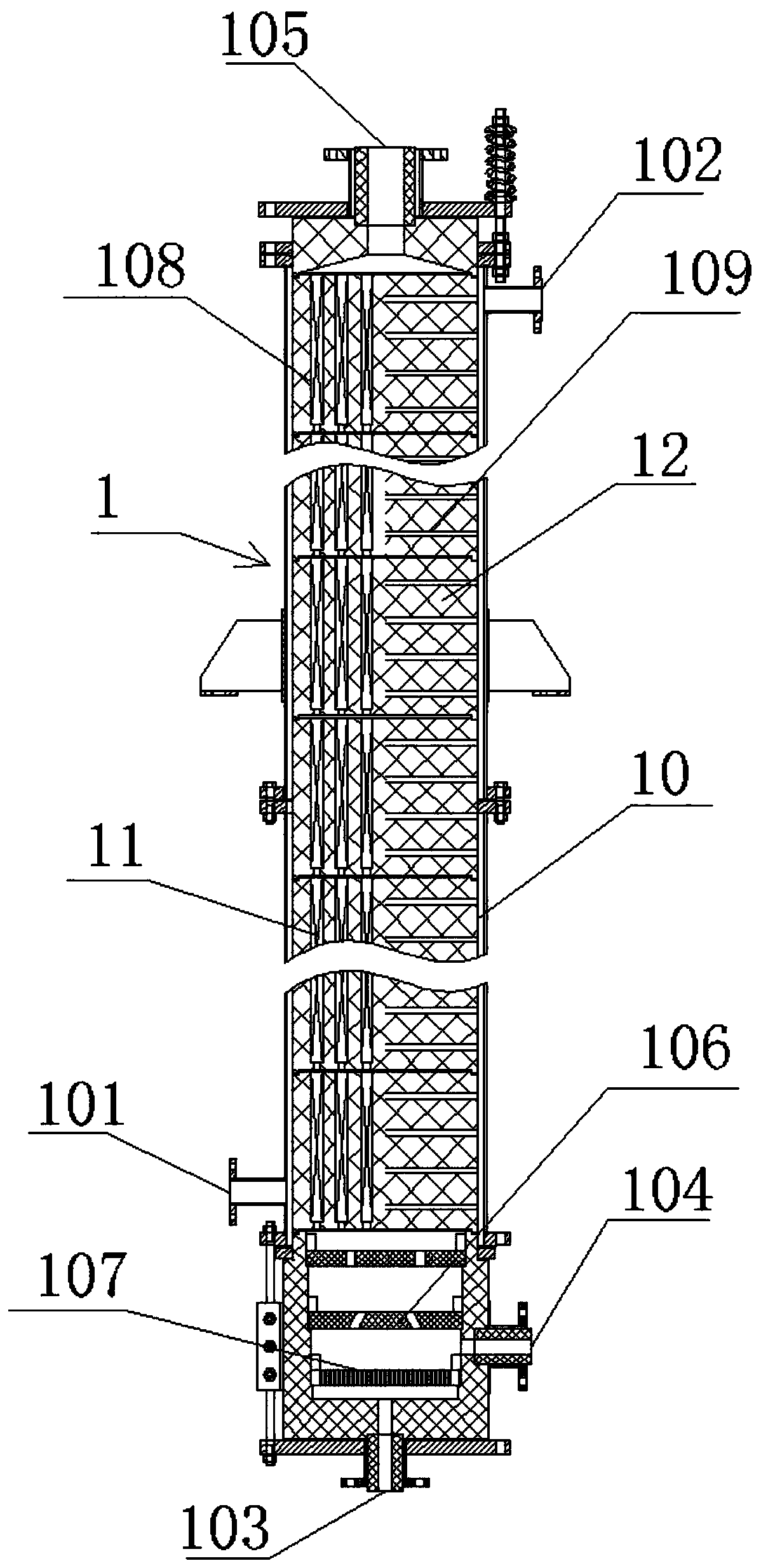
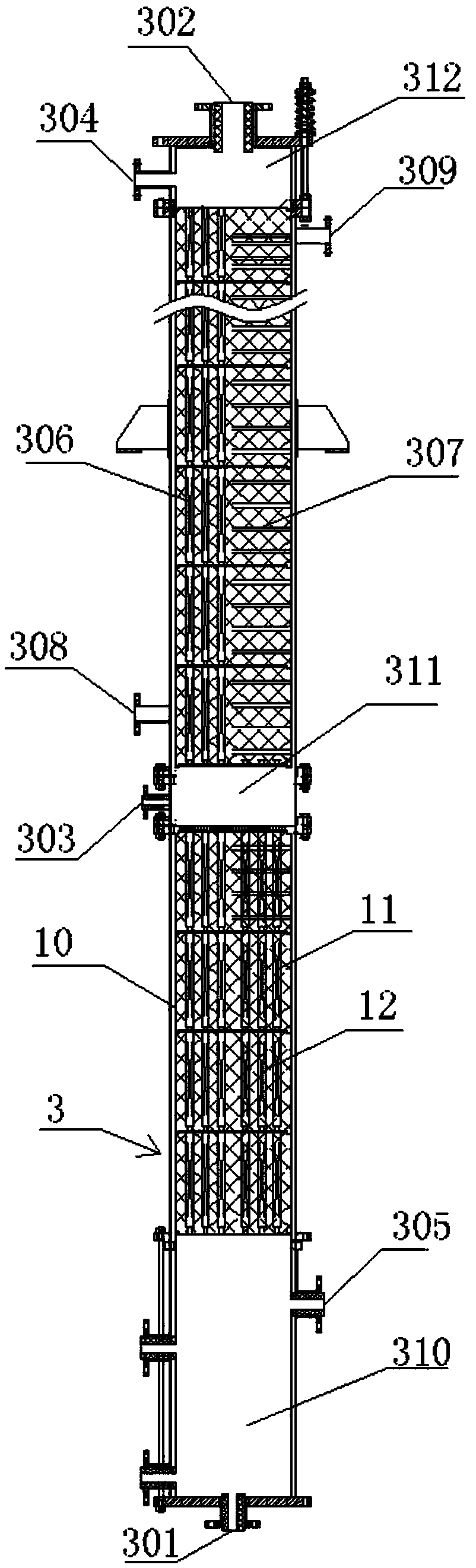
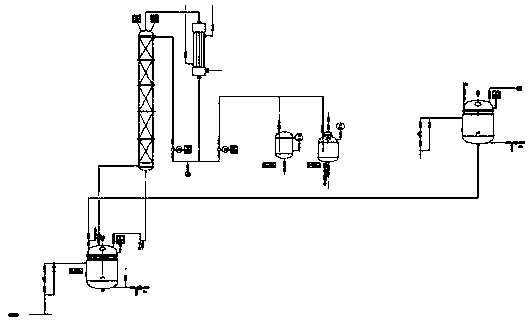
Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method
ActiveCN102952010BHigh yieldPreparation from carboxylic acid anhydridesCarboxylic preparation by ozone oxidationReaction temperatureNickel alloy
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for catalytic synthesis of cinnamic acid by water-soluble calixarene phenolate
InactiveCN108164410ALow yieldLow costOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from carboxylic acid anhydridesAcetic anhydrideBenzaldehyde
The invention is a method for catalytic synthesis of cinnamic acid by water-soluble calixarene phenolate, belonging to the technical field of preparation of organic compounds. The method comprises thefollowing steps: with the water-soluble calixarene phenolate as a catalyst, allowing the water-soluble calixarene phenolate to catalyze benzaldehyde and acetic anhydride to generate a perkin reaction, or catalyze benzaldehyde and propane diacid to generate a Knoevenagel reaction so as to prepare the cinnamic acid, wherein the calixarene phenolate can be repeatedly utilized for a plurality of times. According to the invention, the calixarene phenolate is calixarene phenolate sodium(possitium) salt which is formed through a reaction of calix[n]arene sulfonic acid (n is equal to 4, 6 and 8) andthiocalix[4]arene sulfonic acid with NaOH, KOH, Na2CO3 and K2CO3; and the usage amount of the calixarene phenolate accounts for 5 to 10% of the mass of the benzaldehyde. In the perkin reaction, the mass ratio of the benzaldehyde to the acetic anhydride is 1: 1 to 1: 4; the temperature of the reaction is 140 to 170 DEG C; and the time of the reaction is 1 to 2 h. In the Knoevenagel reaction, the mass ratio of the benzaldehyde to the propane diacid is 1: 1 to 1: 4; the temperature of the reaction is 120 to 150 DEG C; and the time of the reaction is 1 to 2 h. The process of the reaction does notgenerate three industrial wastes like waste gas, waste water and waste residue and is a green chemical process, so the pollution to the environment is reduced; and after completion of the reaction, the product is easily to be separated from the catalyst. The method provided by the invention has the advantages of simple process, high yield, environmental friendliness and low cost, and is a method applicable to industrial production.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
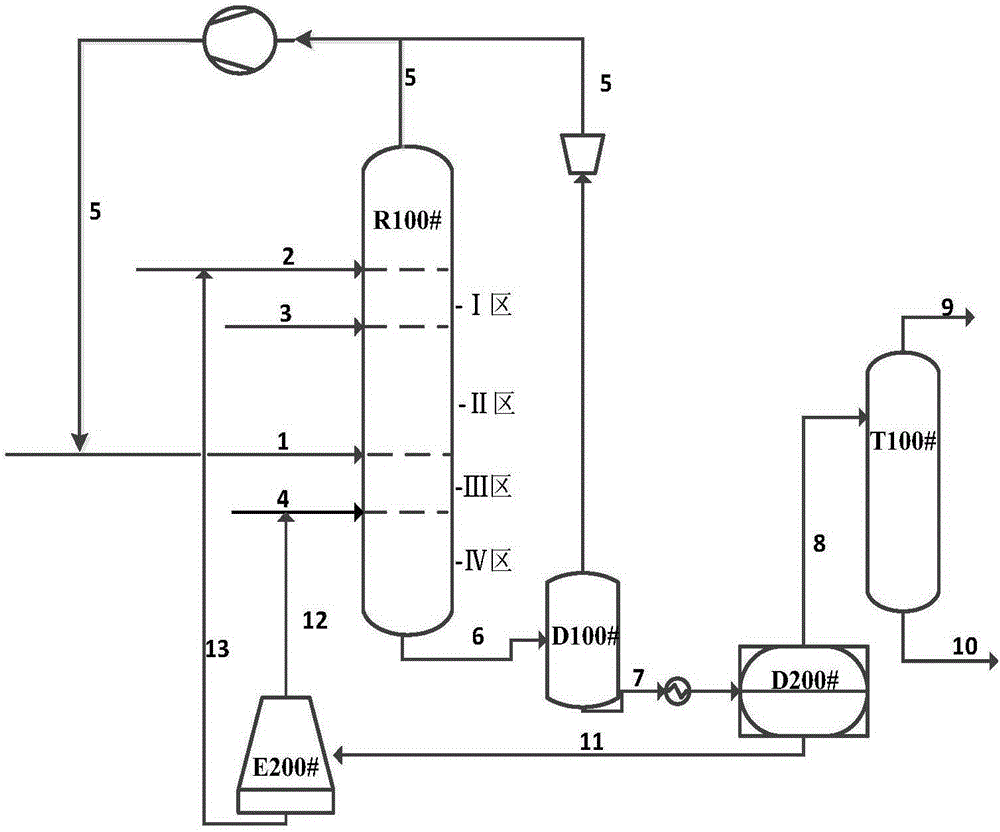
Method for the continuous production of unsaturated carboxylic acid anhydrides
ActiveCN101466660AOrganic compound preparationCarboxylic acid esters preparationOrganic groupReaction zone
The invention relates to a method for the continuous production of unsaturated carboxylic acid anhydrides of formula (I) R-C(O)-O-C(O)-R (I), wherein R is an unsaturated organic group with 2 to 12 C atoms, by anhydride exchange interaction of an aliphatic carboxylic acid anhydride with a carboxylic acid of formula (II) R-COOH (II), wherein R is defined as above, in a rectification column having an upper, a middle and a lower zone. The method is characterized in that f) an inert boiling oil is present in the sump of the column, g) the feed-stock is fed to a reaction zone in stoichiometric ratios, h) the carboxylic acid formed as a by-product is removed at the top of the column, i) the non-reacted feed-stock is returned to the reaction zone, and j) the product of formula (I) is obtained via a lateral outlet, preferably between the middle and the lower column zone.
Owner:EVONIK OPERATIONS GMBH
Cage-shaped cyclobutanoic dianhydrides and process for production thereof
ActiveUS20090012318A1Good light transmissionImprove heat resistancePreparation rom asymmetrical anhydridesOrganic compound preparationOrganic acidHydrogen
A process which comprises reacting a 1,2,3,4-cyclobutanetetracarboxylic-1,2:3,4-dianhydride [1] with an alcohol [2] in the presence of an acid catalyst to obtain a compound [3], isomerizing the compound [3] with a base catalyst into a compound [4], reacting the compound [4] with an organic acid to obtain a compound [5], and reacting the compound [5] with a dehydrating agent to obtain a 1,2,3,4-cyclobutanetetracarboxylic-1,3:2,4-dianhydride: wherein R1 and R2 are each independently hydrogen, halogeno, alkyl of 1 to 10 carbon atoms, halogenated alkyl of 1 to 10 carbon atoms, cycloalkyl of 3 to 8 carbon atoms, phenyl, or cyano; and R3 is alkyl of 1 to 10 carbon atoms.
Owner:NISSAN CHEM IND LTD
Calcium carbonate modifier and preparation method thereof
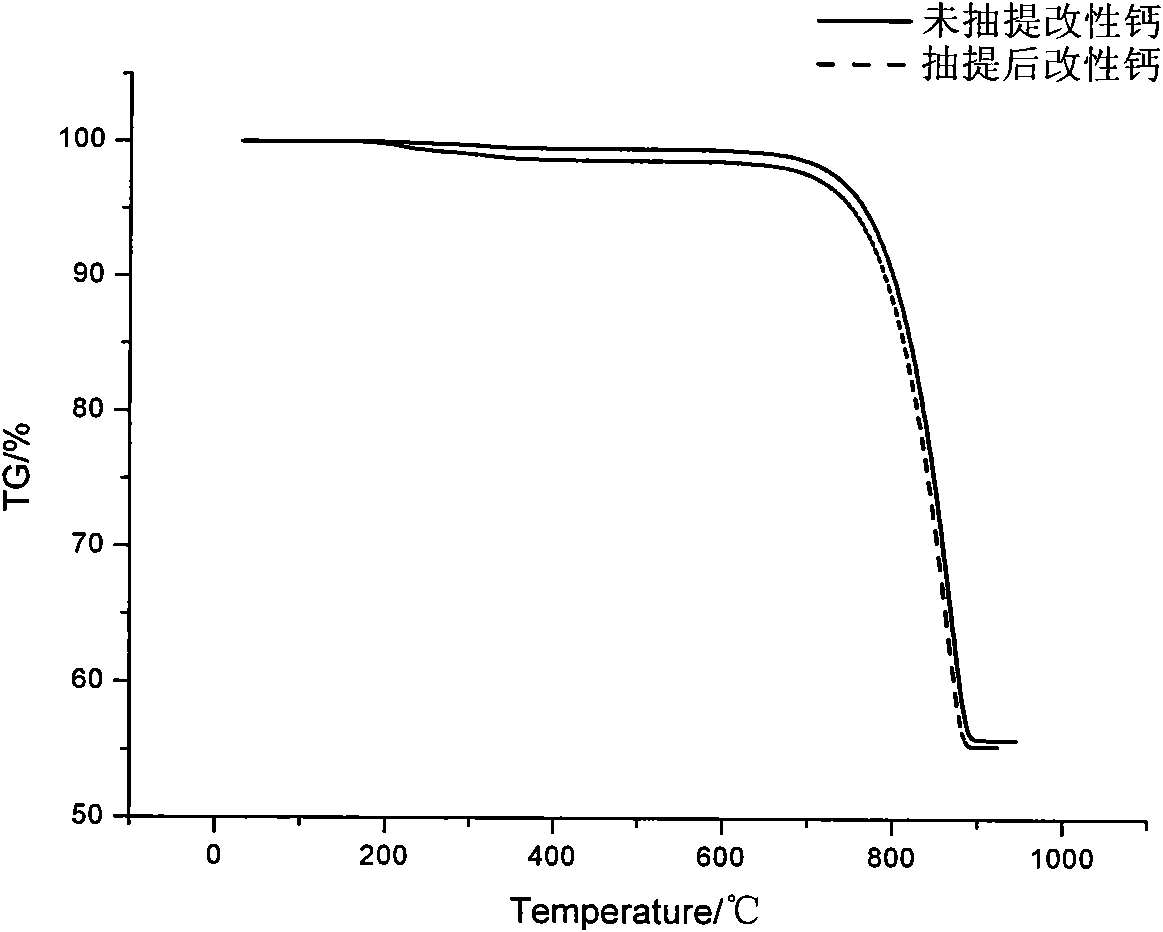
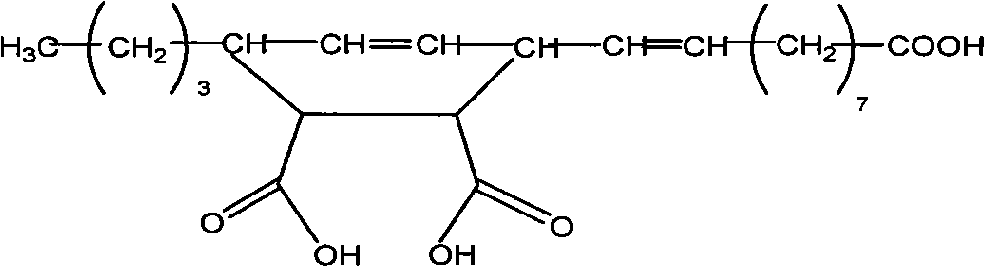
InactiveCN101671495AGood compatibilityImprove interface performancePreparation from carboxylic acid anhydridesPigment treatment with non-polymer organic compoundsHydrolysateStearic acid
The invention provides a calcium carbonate modifier and a preparation method thereof. The calcium carbonate modifier is eleostearic acid estolide hydrolysate, which is prepared by taking eleostearic acid and maleic anhydride as materials to directly synthesize eleostearic acid estolide by one-step procedure and using a proper amount of water to hydrolyze the eleostearic acid estolide. The invention has the advantages of simple production process, low production cost, high yield, easy popularization and the like. The calcium carbonate modifier has more action points and can chemically react with calcium carbonate, thereby having better effect than the traditional stearic acid modifier.
Owner:ZHANGJIAJIE HENGLIANG MINING
Synthesis method for 3,3',4,4'-biphenyltetracarboxylic acid
ActiveCN110818551AEasy to handleReduce manufacturing costOrganic compound preparationPreparation from carboxylic acid anhydridesGlutaric anhydridePolymer science
The invention discloses a synthesis method for 3,3',4,4'-biphenyltetracarboxylic acid. The synthesis method comprises the following steps: with 4,4'-dimethyl biphenyl as a starting raw material, subjecting the 4,4'-dimethyl biphenyl and cyclic anhydride as shown in a formula I which is described in the specification to a Friedel-Crafts acylation reaction so as to obtain an intermediate II, and carrying out an oxidation reaction so as to obtain the 3,3',4,4'-biphenyltetracarboxylic acid, wherein the cyclic anhydride as shown in the formula I is selected from the group consisting of succinic anhydride, glutaric anhydride, adipic anhydride and maleic anhydride. The method provided by the invention does not need an expensive catalytic system, so the production cost of synthesizing the 3,3',4,4'-biphenyltetracarboxylic acid is greatly reduced; the method provided by the invention is simple to operate, mild in reaction conditions, less in three wastes, easy in wastewater treatment and friendly to the environment; and the raw material, namely the 4,4'-dimethyl biphenyl adopted in the method provided by the invention is a byproduct of a medical intermediate, namely sartan biphenyl, and theproduction amount of the byproduct accounts for about 10% of the production amount of the sartan biphenyl, so the waste can be turned into treasure after the byproduct is applied to synthesis of the3,3',4,4'-biphenyltetracarboxylic acid.
Owner:CHANGZHOU SUNLIGHT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
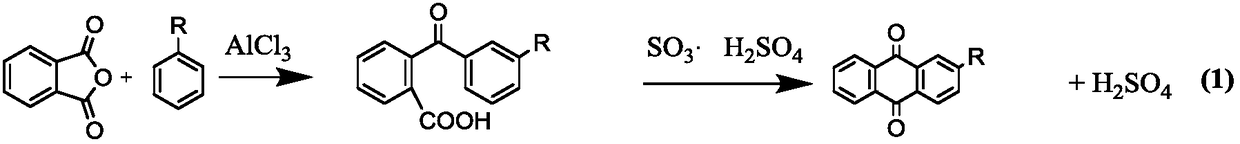
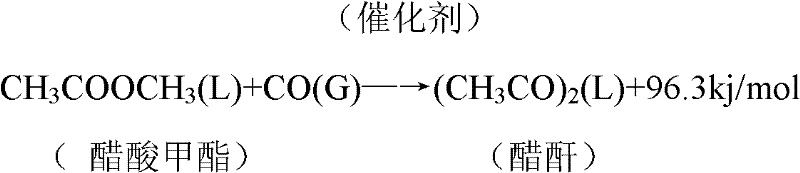
![Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method](https://images-eureka.patsnap.com/patent_img/e56dd87e-6ee2-4c81-a5ff-38f34d499a5e/HDA00002338829700011.PNG)
![Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method](https://images-eureka.patsnap.com/patent_img/e56dd87e-6ee2-4c81-a5ff-38f34d499a5e/HDA00002338829700012.PNG)
![Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method Bicyclo[2.2.1]heptane-2,3-dicarboxylic acid disodium preparation method](https://images-eureka.patsnap.com/patent_img/e56dd87e-6ee2-4c81-a5ff-38f34d499a5e/HDA00002338829700021.PNG)