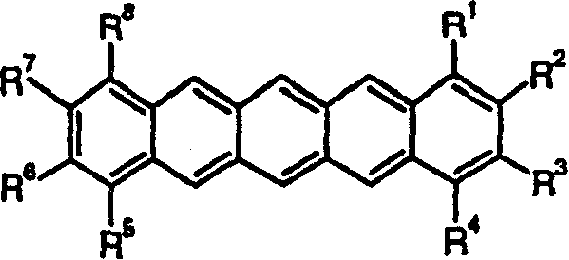
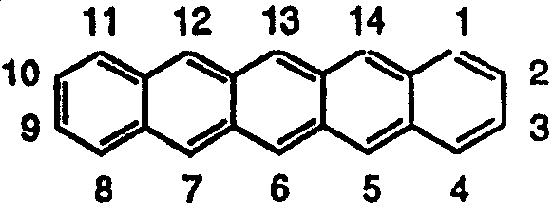
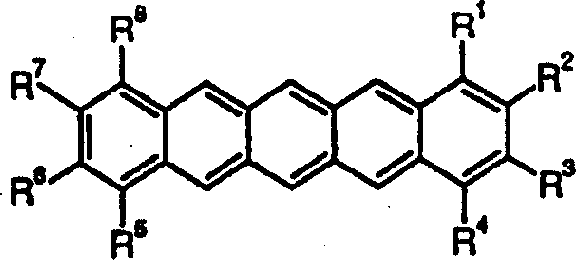
Substituted pentacene semiconductors
A kind of pentacene and substituent technology, applied in the field of organic compounds, can solve problems such as inability to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the preparation of 2,9-dihexylpentacene
[0076] Preparation of 2,5-di(4-hexylbenzoyl)terephthalic acid
[0077] In 3.5 hours, to the mixture of 25.7g aluminum chloride, 51.3mL of 1,2-dichloroethane and 10g of benzene-1,2,4,5-tetracarboxylic dianhydride (pyrellitic dianhydride) While cooling, add 14.9 g of hexylbenzene and 6.40 g of diisopropylethylamine in 25 mL of 1,2-dichloroethane solution, keeping the temperature between 15°C and 20°C. After the addition was complete, the resulting mixture was stirred for an additional 15 minutes, then heated to 40° C. for 1 hour. The hot mixture was poured into a beaker containing 200 g of ice cubes and 75 mL of concentrated hydrochloric acid and stirred overnight at room temperature. The water phase was poured out, and the obtained oily solid was stirred with 500 mL of water, and then the water was poured out. Such water washes were repeated, and the resulting residue was dissolved in 250 mL of acetone and conc...
Embodiment 2
[0084] Embodiment 2: Preparation of 2,9-dinonylpentacene
[0085] Preparation of 2,5-bis(4-nonylbenzoyl)terephthalic acid
[0086]A mixture of 1370g of aluminum chloride, 533.7g of benzene-1,2,4,5-tetracarboxylic dianhydride and 2750mL of 1,2-dichloroethane was stirred at 15°C within 3.5 hours, and 341.5 gram of N,N-diisopropylethylamine in 1334 mL of 1,2-dichloroethane solution, the temperature was maintained at 15°C to 20°C. The reaction mixture was stirred overnight at room temperature. This mixture was added to a mixture of 2500 g of ice and 2500 mL of concentrated hydrochloric acid with good stirring. The mixture was divided into 800 mL portions and the following procedure was followed for each portion. To 800 mL of this mixture was added 800 mL of tetrahydrofuran, 800 mL of ethyl acetate, and 800 mL of water. The mixture was stirred and the phases were separated. The organic phase was filtered and the filtrate was concentrated in vacuo. The residues were combined...
Embodiment 3
[0093] Embodiment 3: Preparation of 2,9-dilauryl pentacene
[0094] Preparation of 2,5-bis(4-dilaurylbenzoyl)terephthalic acid
[0095] To a mixture of 492 g of aluminum chloride and 988 mL of 1,2-dichloroethane was added 192 g of benzene-1,2,4,5-tetracarboxylic dianhydride (pyrellitic dianhydride). The resulting mixture was cooled to 16 °C and a solution of 434 g of 1-laurylbenzene, 123 g of diisopropylethylamine and 480 mL of 1,2-dichloroethane was added within 3.5 hours, maintaining the temperature at 15 °C to between 20°C. The mixture was stirred overnight at room temperature and poured into a beaker with 1000 g of ice and 1000 g of concentrated hydrochloric acid. The mixture was stirred for 1 hour, and the water in the coagulum was poured off. The mixture was divided into 800 mL portions and the following procedure was followed for each portion. To 800 mL of the mixture were added 800 mL of tetrahydrofuran, 800 mL of ethyl acetate, and 800 mL of water. The mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com