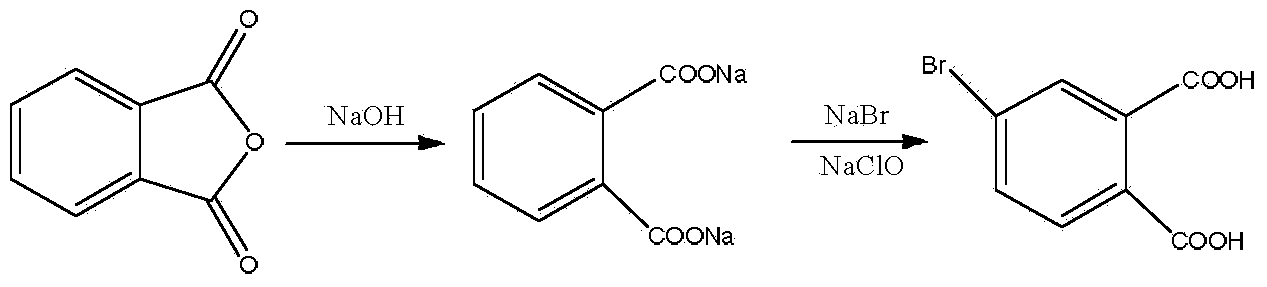
Preparation method of 4-bromophthalic acid
A technology of phthalic acid and phthalic anhydride, which is applied in the field of preparation of 4-bromophthalic acid, can solve the problems of easily polluted environment, complicated operation, low utilization rate of bromine, etc., and achieves good selectivity , the effect of high yield and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Slowly add 148g of phthalic anhydride and 40g of sodium hydroxide to 690g of water under ultrasound, place in a 2000ml four-necked reaction flask with a condenser and a thermometer, then add 106g of sodium bromide, and mix well by ultrasound. Heat to 55° C., and slowly drop in an aqueous solution containing 77 g of sodium hypochlorite (concentration: 2 mol / L) under ultrasound, while adjusting the pH value between 5-8 with 5 wt % dilute hydrochloric acid. After feeding, keep warm for 1 hour, then raise the temperature to 80°C for 2 hours. Cool to 2° C., treat under ultrasonic for 0.5 hour, let stand, and filter to obtain 236.2 g of crude product (HPLC purity: 92%).
[0026] The crude product was recrystallized with pure water to obtain 198.3 g of 4-bromophthalic acid, the content detected by HPLC was 98.9%, and the yield was 81%.
[0027] Add hydrochloric acid to the filtrate to acidify to about pH2, cool down, and recover unreacted phthalic acid.
Embodiment 2
[0029] Slowly add 100g of phthalic anhydride and 26g of sodium hydroxide into 600g of water successively under ultrasound, place in a 1000ml four-necked reaction flask with a condenser tube and a thermometer, then add 77g of sodium bromide, and mix well by ultrasound. Heat to 65°C, slowly drop into aqueous solution containing 50g of sodium hypochlorite (concentration: 3mol / L) under ultrasound, and adjust the pH value between 5-8 with 10wt% dilute hydrochloric acid at the same time. After feeding, keep warm for 2 hours, then raise the temperature to 90°C for 1 hour. Cool to -2°C, treat with ultrasound for 1 hour, let stand, and filter to obtain 161.5 g of crude product (HPLC purity: 93%).
[0030] The crude product was recrystallized with pure water to obtain 137.3 g of 4-bromophthalic acid, the content detected by HPLC was 98.6%, and the yield was 83%.
[0031] Add hydrochloric acid to the filtrate to acidify to about pH2, cool down, and recover unreacted phthalic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com