Method for the continuous production of unsaturated carboxylic acid anhydrides
An unsaturated and carboxylic acid anhydride technology, applied in the field of continuous preparation of unsaturated carboxylic acid anhydrides, can solve problems such as the inability to completely remove by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of methacrylic anhydride
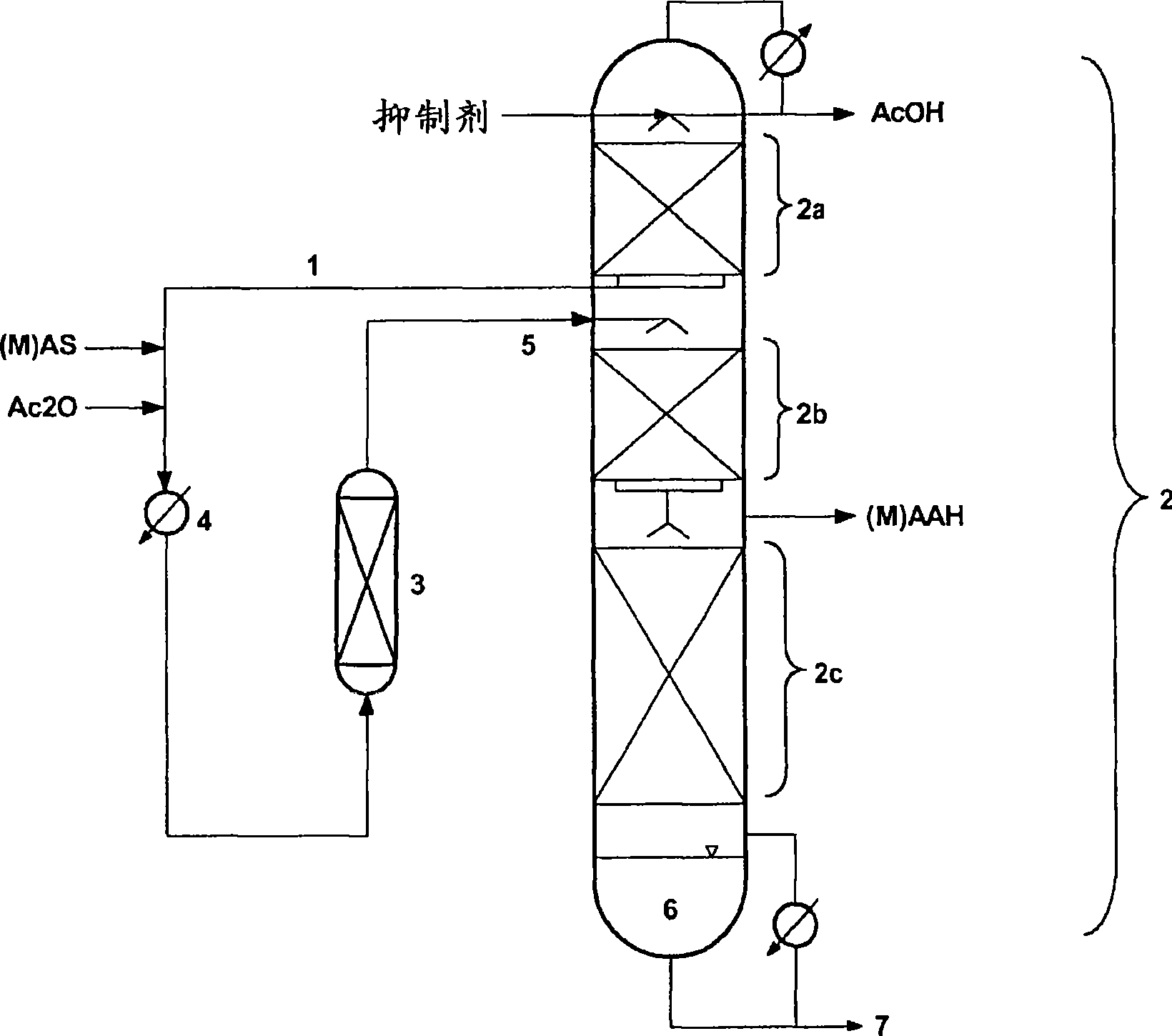
[0058] For the preparation of methacrylic anhydride by the reaction of methacrylic acid with acetic anhydride, the assembly is according to figure 1 test device. The rectification column ( 2 ) has a total of about 35 separation stages (15 in the upper zone ( 2a ), 12 in the middle zone ( 2b ) and 8 in the lower zone ( 2c )). The columns were 5.5 m high with an internal diameter of 100 mm and were assembled with structured packings of type CY from the company Sulzer (areas 2a and 2b) and BSH 400 from the company Montz (area 2c) with intermediate connections (Zwischenstück) and bottoms. Phenothiazines are used as polymerization inhibitors. The pressure at the top of the column was 20 mbar. A temperature profile of 164° C. (bottom) to 23° C. (top) was set under static conditions. The removal of acetic acid at the top of the column and methacrylic anhydride at the side outlet (between regions 2b and 2c ) and the h...
Embodiment 2
[0063] Embodiment 2: the preparation of acrylic anhydride
[0064] For the preparation of acrylic anhydride by the reaction of acrylic acid with acetic anhydride, the same experimental setup as described in Example 1 was used.
[0065] The pressure at the top of the column, the reaction temperature and the recycle flow are practically the same as those described in Example 1. The same reactor setup, the same polymerization inhibitor, the same catalyst (type and amount) and the same boiling oil (type and amount) were also used. A temperature profile of 167° C. (bottom) to 23° C. (top) was set under static conditions.
[0066] 1500 g / h of acetic anhydride and 2118 g / h of acrylic acid were continuously metered in freshly.
[0067] 1712 g / h of acetic acid are produced overhead. 1797 g / h of acrylic anhydride with a purity of 99.7% (GC-analysis) were withdrawn at the side stream draw. The yield of acrylic anhydride was 97% based on the acetic anhydride used or the acrylic acid u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com