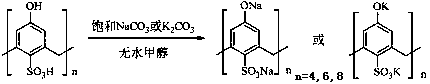
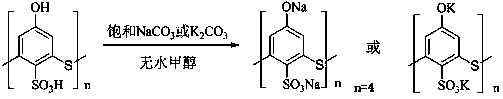Method for catalytic synthesis of cinnamic acid by water-soluble calixarene phenolate
A water-soluble, cinnamic acid technology, applied in chemical instruments and methods, catalytic reaction, preparation of carboxylic acid by ozone oxidation, etc., can solve problems such as inability to recycle, difficult separation, and no reports, and achieves the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Calix[4]arene phenol sodium salt catalyzed Perkin reaction to synthesize cinnamic acid: add freshly distilled benzaldehyde 1.5ml (15 mmol), acetic anhydride 4.0 ml (42 mmol) and 0.69 g calix[4]arene phenol Sodium salt, according to the installation of condenser, thermometer, magnetic stirring. After reacting at 170 °C for 2 h, the catalyst was removed by filtration and washed with 10% Na 2 CO 3 The pH of the solution was adjusted to 9, and the aqueous phase was extracted twice with 10 mL of ethyl acetate. The aqueous phase was filtered with a small amount of activated carbon to absorb impurities, and the filtrate was adjusted to pH = 3 to 4 with concentrated hydrochloric acid. A large number of white crystals were precipitated, filtered with suction, and dried. , weighing, the productive rate is 62.6%.
Embodiment 2
[0024] Calix[6]arene phenol sodium salt catalyzed Perkin reaction to synthesize cinnamic acid: add freshly distilled benzaldehyde 1.5ml (15 mmol), acetic anhydride 4.0 ml (42 mmol) and 1.04g calix[6]arene phenol Sodium salt, install a condenser, a thermometer, and magnetically stir. After reacting at 160 °C for 2 h, the catalyst was removed by filtration and washed with 10% Na 2 CO 3 Adjust the pH of the solution to 9, then extract the water phase twice with 10 mL of ethyl acetate, use a small amount of activated carbon to absorb impurities in the water phase, and filter with suction, adjust the filtrate to pH = 3 to 4 with concentrated hydrochloric acid, and precipitate white crystals, filter with suction, dry, Weighed, the yield was 55.0%.
Embodiment 3
[0026] Calix[8]arene phenol sodium salt catalyzed Perkin reaction to synthesize cinnamic acid: add freshly distilled benzaldehyde 1.5ml (15 mmol), acetic anhydride 5.7 ml (60 mmol) and 1.38g calix[8]arene phenol Sodium salt, equipped with a condenser, a thermometer, and magnetic stirring. The reaction was completed after 2 h at 150 °C, the catalyst was removed by filtration, and 10% Na 2 CO 3 Adjust the pH of the solution to 10, then extract the water phase twice with 10 mL of ethyl acetate, use a small amount of activated carbon to absorb impurities in the water phase, and filter with suction, adjust the filtrate to pH = 3 to 4 with concentrated hydrochloric acid, a large number of white crystals are precipitated, filter with suction, and dry , weighing, the productive rate is 51.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com