Functionalized column aromatic hydrocarbon derivative and preparation method thereof
A technology for pillar aromatics and derivatives, which is applied in the field of derivatives and corresponding preparations, and can solve the problems of undeveloped means of functionalizing pillar aromatics and limiting the application of functionalized pillar aromatics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The synthetic route of embodiment 1 compound 1-2:
[0115]
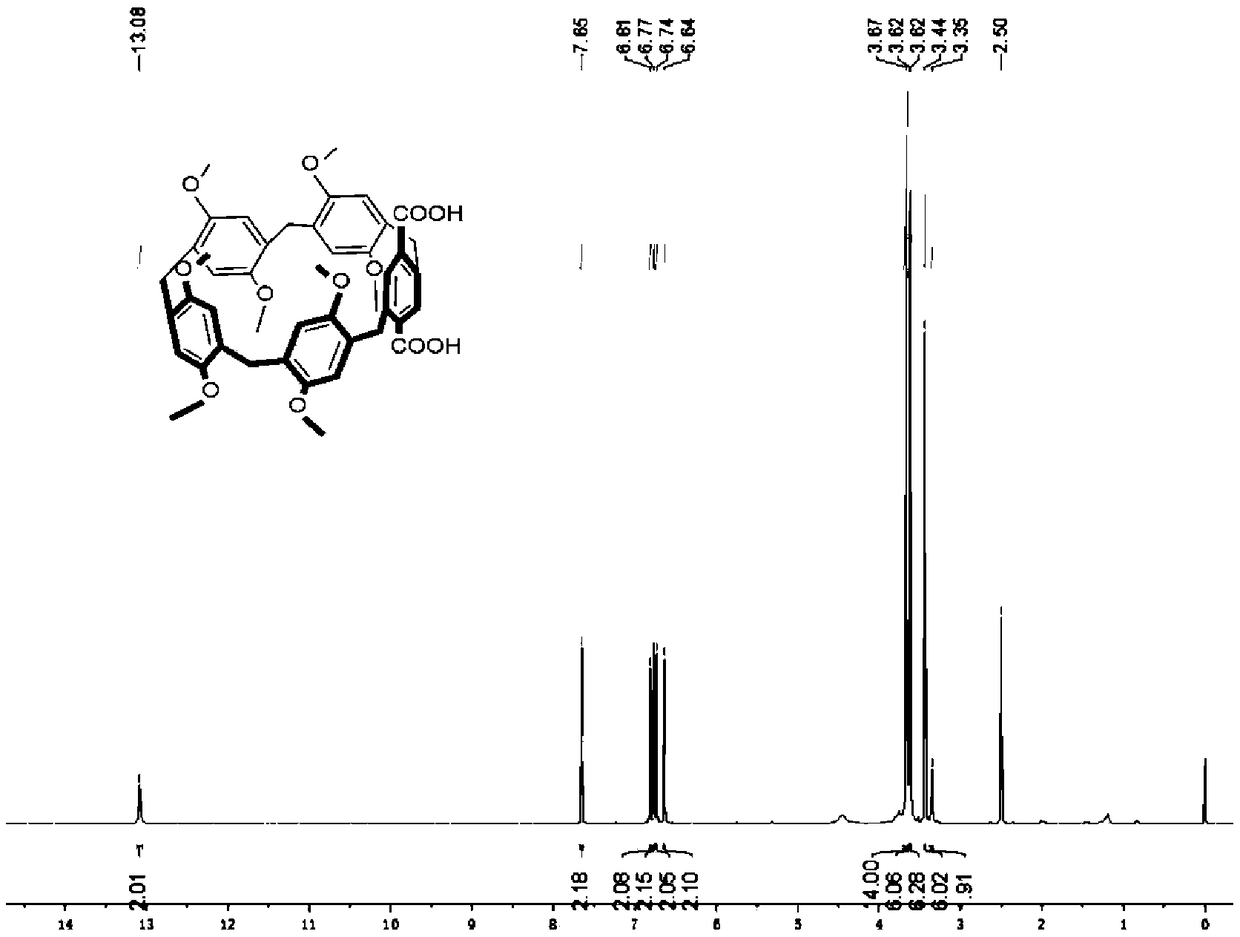
[0116] (1) Synthesis of compound A-1: p-xylylene dimethyl ether (1.38g, 10mmol), paraformaldehyde (300mg, 10mmol) and boron trifluoride ether (1.25ml, 10mmol) were added to 25ml of 1 , 2-dichloroethane, stirred at room temperature for 40min. The color of the reaction solution changed from cloudy white to dark green. Thin-layer chromatography (Thin-layer chromatography, TLC) board confirms that the reaction is complete, the reaction solution is washed twice with 20ml water, the organic layer is concentrated under reduced pressure to obtain a crude product, which is then dissolved in dichloromethane and mixed with silica gel powder, and separated by column chromatography to obtain Pure white solid A-1 (1 g, yield 60%). 1 H-NMR (500MHz, CDCl 3 , 298K): δ6.84(s, 10H), 3.76(s, 10H), 3.71(s, 30H). 13 C-NMR (CDCl 3 , 67.5MHz, ppm): 6150.4, 128.0, 133.6, 55.5, 29.5. ESI-HRMS (electrospray-high resolution mas...
Embodiment 2
[0129] The synthetic route of embodiment 2 compound 1-4 is as follows:
[0130]
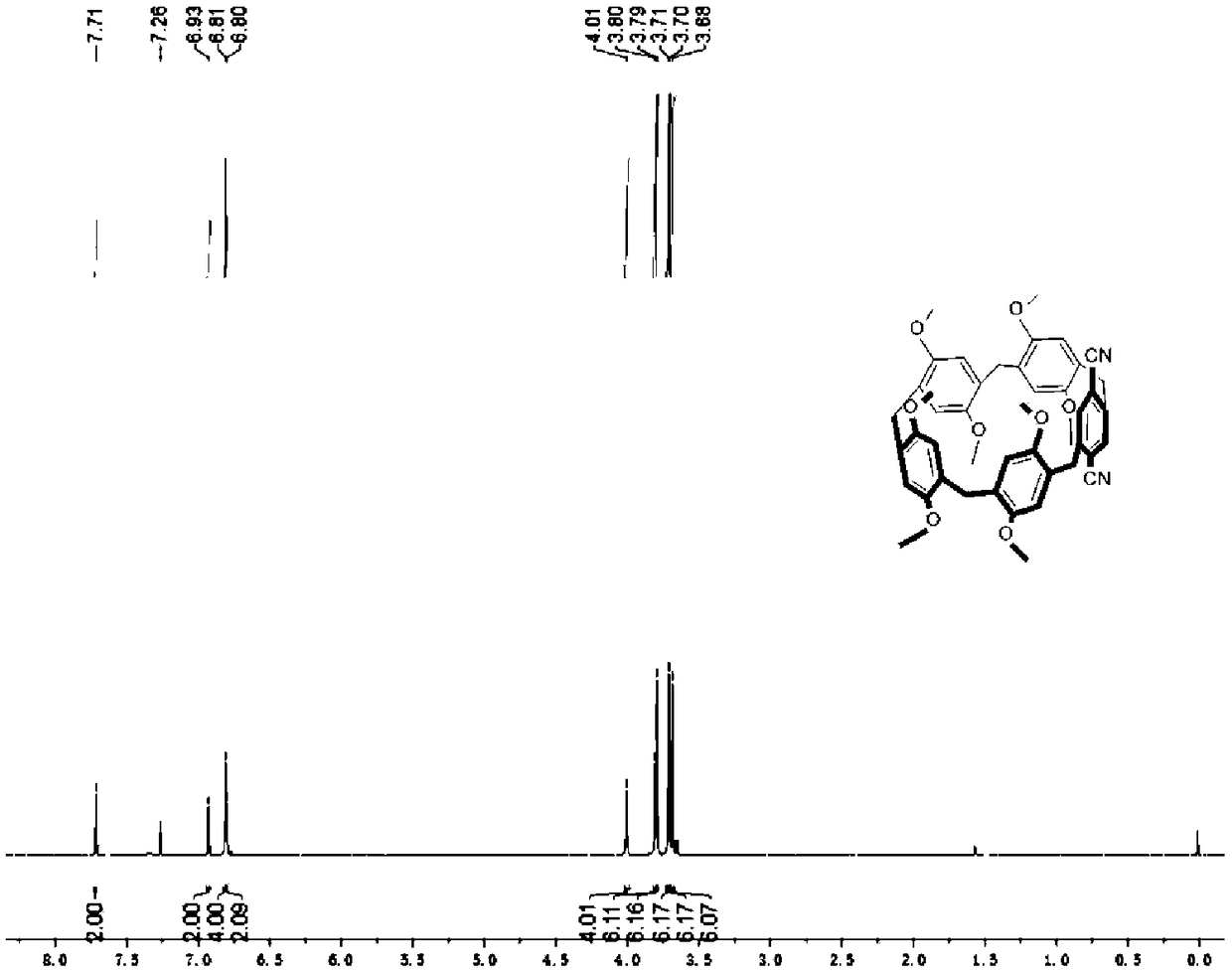
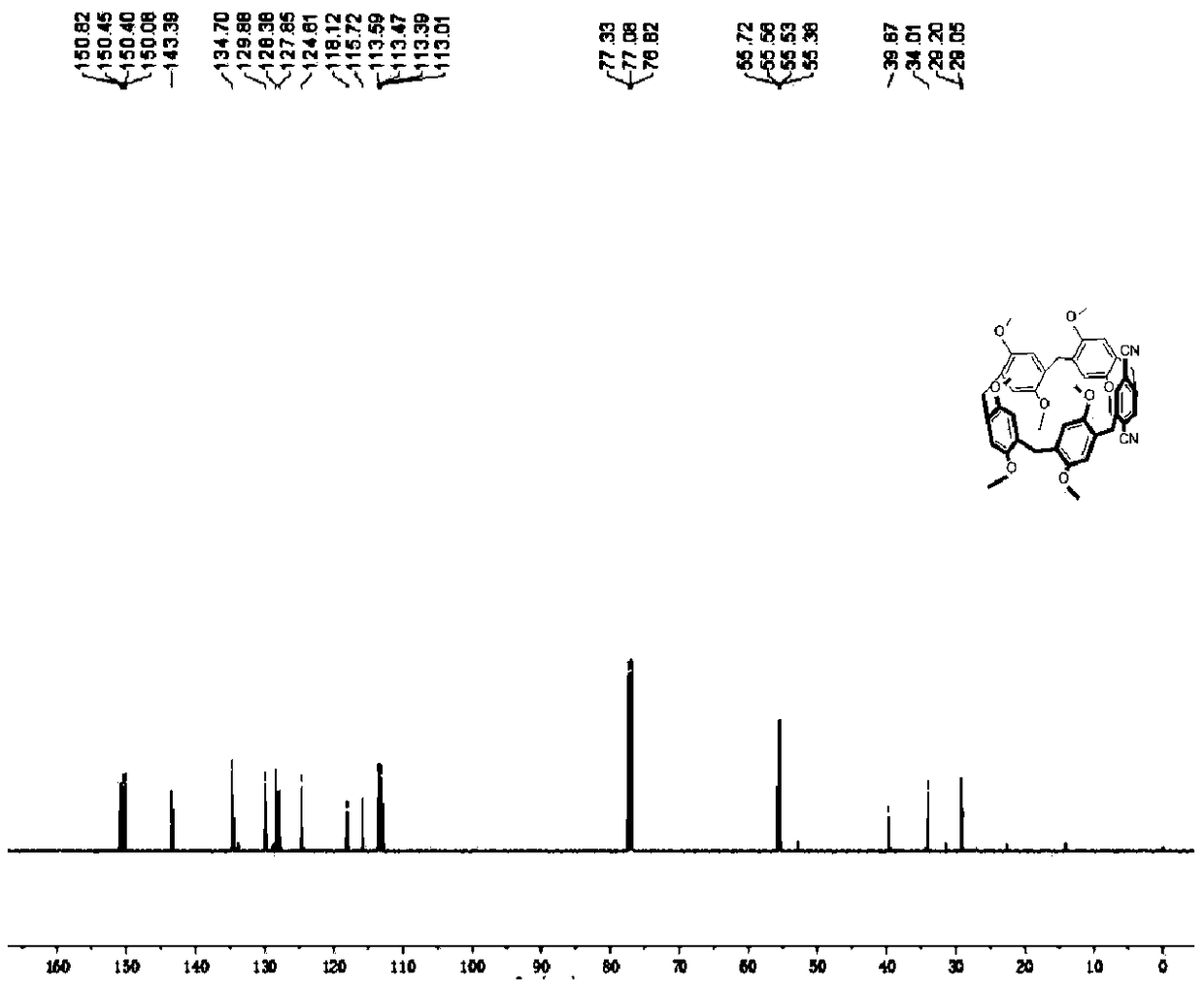
[0131] Its preparation method can refer to I-2. Wherein, the molar equivalent ratio of step (2) A-1 to ammonium cerium nitrate is 1:4, and the reaction time is 10 min. Wherein, the molar equivalent ratio of step (3) B-2 to sodium borohydride is 1:5, and the stirring time is 10 min. Wherein, the molar equivalent ratio of step (4) C-2 to base and trifluoromethanesulfonic anhydride is 1:6:6, and the reaction time is 10 h. Wherein, the molar equivalent ratio of step (5) D-2, zinc cyanide and catalyst is 1:4:0.2. The binding constant of the target compound I-3 with 1,6-adiponitrile (deuterated chloroform, 25°C) is 2.1±0.3M after nuclear magnetic titration test -1 .
[0132] see Figure 5-8 , Figure 5 for the target compound I-3 1 H-NMR spectrum; Figure 6 for the target compound I-3 13 C-NMR spectrum; Figure 7 For the target compound I-4 1 H-NMR spectrum; Figure 8 For the target comp...
Embodiment 3
[0133] The synthetic route of embodiment 3 compound 1-6 is as follows:
[0134]
[0135] Its preparation method can refer to I-2. Wherein, the molar equivalent ratio of step (2) A-1 to ammonium cerium nitrate is 1:6, and the reaction time is 20 minutes. Wherein, the molar equivalent ratio of step (3) B-3 to sodium borohydride is 1:7.5, and the stirring time is 15 min. Wherein, the molar equivalent ratio of step (4) C-3 to base and trifluoromethanesulfonic anhydride is 1:9:9, and the reaction time is 10 h. Wherein, the molar equivalent ratio of step (5) D-3, zinc cyanide and catalyst is 1:6:0.3.
[0136] see Figure 9-12 ,in Figure 9 For the target compound I-5 1 H-NMR spectrum; Figure 10 for 3 target compounds I-5 13 C-NMR spectrum; Figure 11 for the target compound I-6 1 H-NMR spectrum; Figure 12 It is the high resolution mass spectrum of the target compound I-6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com