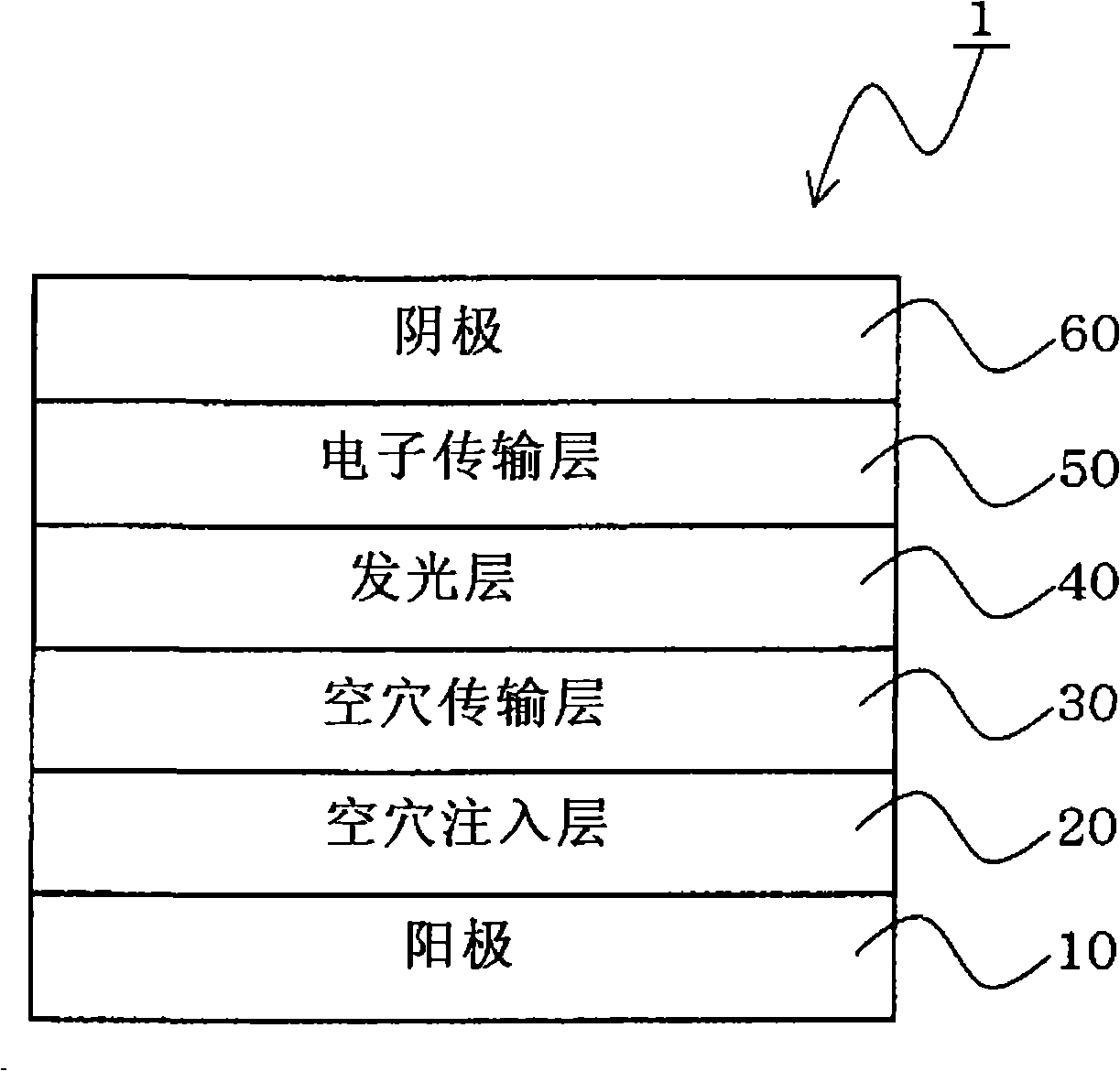
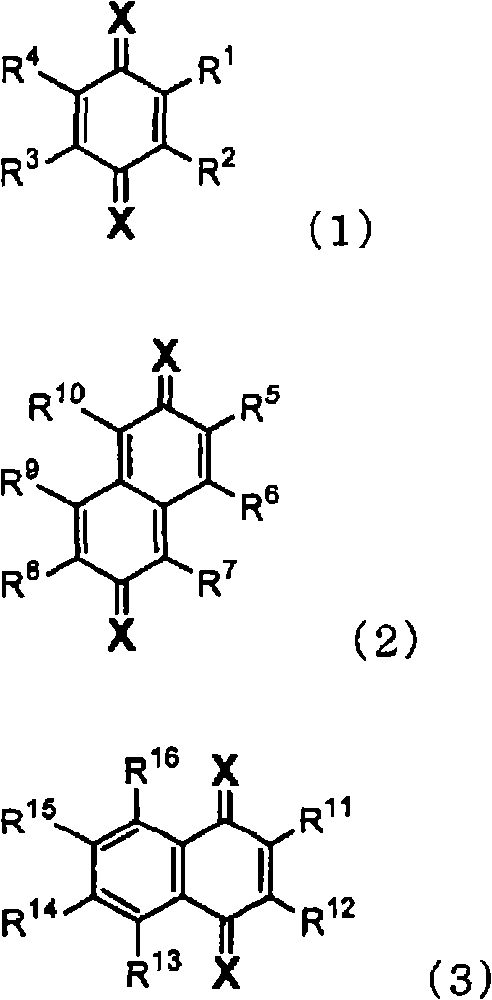
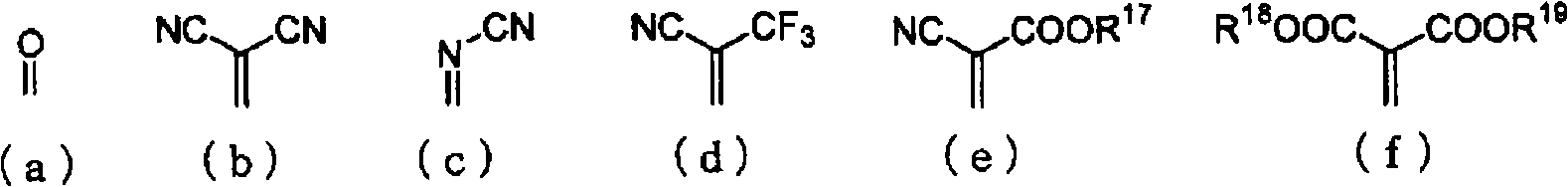Material for organic electroluminescent device and organic electroluminescent device using the same
An electroluminescence element and an electroluminescence technology, which are applied in the field of materials for organic electroluminescence elements and organic electroluminescence elements, and can solve the problems of small molecular weight, insufficient heat resistance stability, and shortened life span.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0287] Synthesis of (A-19)
[0288] 4.5 g of potassium tert-butoxide and 10 mL of DMSO were mixed at room temperature under a nitrogen atmosphere, and 5.2 g of 3-trifluoromethylphenol was added thereto. A solution of 4.3 g of 2,5-dibromobenzoquinone and 15 mL of DMSO was added dropwise to the mixed liquid, followed by stirring at room temperature for 8 hours. Thereafter, ethyl acetate and water were added for layering operation, anhydrous sodium sulfate was added to filter the organic layer, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography to obtain 3.0 g of compound (A-19).
[0289] Measure the IR of the compound, at 1665cm -1 The absorption of the carbonyl group was confirmed. M / Z = 428 was confirmed by mass spectrometry.
[0290] The resulting compound was dissolved in acetonitrile at a concentration of 0.01 mol / L, using tetrabutylammonium perchlorate (TBAP) as the supporting electrolyte, and a satura...
Embodiment 2
[0292] Synthesis of (A-1)
[0293] First, a solution obtained by mixing 1.7 g of (A-19), 0.54 g of malononitrile, and dichloromethane was stirred while cooling with ice under a nitrogen atmosphere, and 2.4 mL of titanium tetrachloride was added dropwise, and then Add 3.6 mL of pyridine dropwise. Then, after stirring for 5 hours, dichloromethane was distilled off under reduced pressure, and 5 mL of 1N hydrochloric acid was added thereto. The precipitate was recrystallized from acetonitrile, followed by sublimation purification to obtain 0.8 g.
[0294] Measure the IR of the compound, at 2222cm -1 Confirm the absorption of cyano group. M / Z = 524 was confirmed by mass spectrometry.
[0295] The resulting compound was dissolved in acetonitrile at a concentration of 0.01 mol / L, using tetrabutylammonium perchlorate (TBAP) as the supporting electrolyte, and a saturated calomel (SCE) electrode as the reference electrode, by cyclic voltaic The reduction potential was determined by...
Embodiment 3
[0297] Synthesis of (B-7)
[0298] In Example 1, 5.0 g of 1,5-dibromo-2,6-naphthoquinone was used instead of 2,5-dibromobenzoquinone, and 5.2 g of 4-trifluoromethylphenol was used instead of 3-trifluoromethyl Phenol was synthesized in the same manner as in Example 1 except that, to obtain 2.7 g of (B-7).
[0299] Measure the IR of the compound, at 1658cm -1 The absorption of the carbonyl group was confirmed. M / Z = 478 was confirmed by mass spectrometry.
[0300]In addition, the reduction potential measured by cyclic voltammetry was 0.01V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com