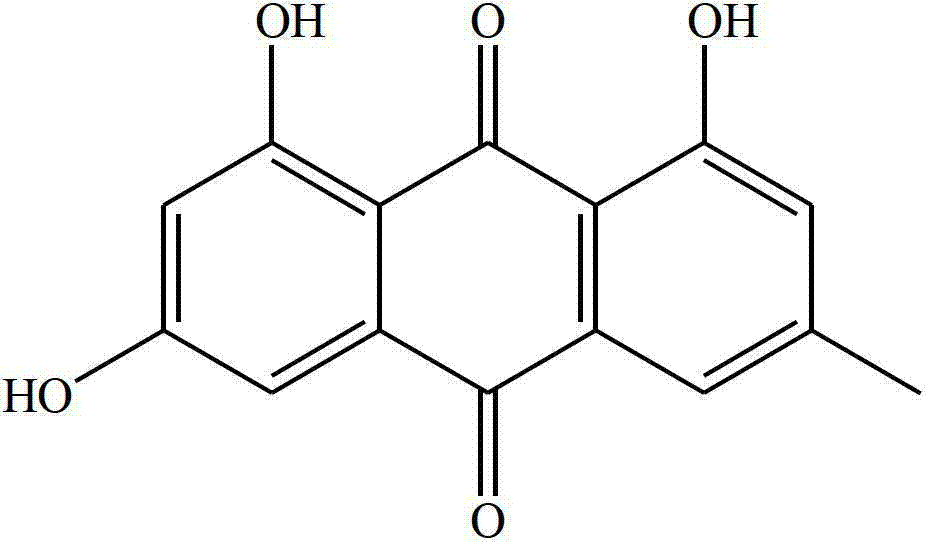
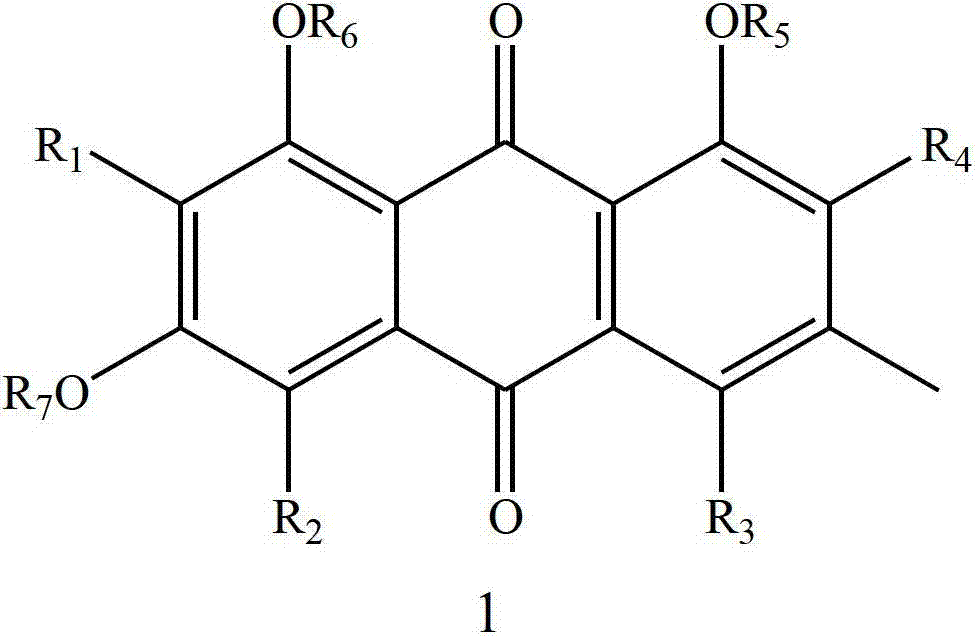
Emodin derivative and application thereof in preparing antibacterial agents
A technology of emodin and derivatives, which is applied in the field of medicine, can solve the problems of not too long clinical application time and increased drug resistance, and achieve good anti-drug-resistant bacteria effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
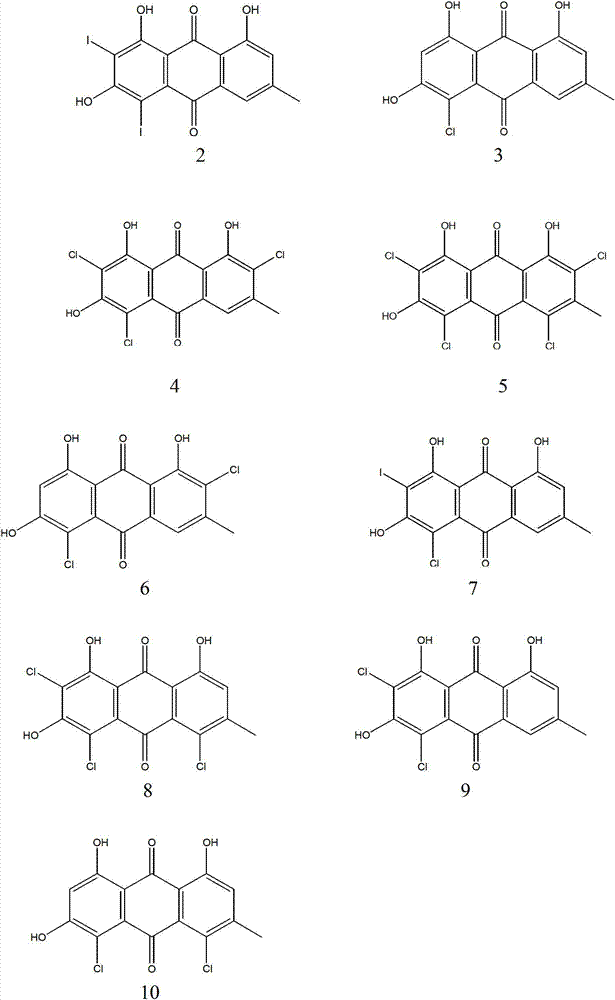
[0023] Example 1 4-chloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (3) and 4,7-dichloro-1,3,8-trihydroxy-6-methanol Synthesis of 9,10-anthraquinone (6), 4,5-dichloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (10)
[0024]
[0025] Take 4.46g (14.8mmol) of emodin, 300ml of anhydrous acetic acid, and 30ml of concentrated hydrochloric acid in an oil bath at 85°C and stir for 10 minutes, mix well, and then add 1.6ml (16mmol) of 30% hydrogen peroxide dropwise:
[0026] 1) Add 8 times in batches, slowly drop 200 μl each time, drop every 5 minutes. After the dropwise addition, continue to stir for 20 minutes to terminate the reaction. After the reaction liquid is cooled, add 250ml of water, and immediately a yellow precipitate is formed. It is filtered under reduced pressure and separated on a silica gel column to obtain compound 3 (4.02g, 13.22mmol, 89%)
[0027] 2) Add all at one time, continue to stir for 20 minutes after the dropwise addition, and then stop the reactio...
Embodiment 2
[0030] Example 2 Synthesis of 2-iodo-4-chloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (7)
[0031]
[0032] Take 0.8 g of 2-iodo-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (synthesized in our laboratory, see literature: Huang Wen, He Yang, Wu Xiaohua. Emodin derivatives and Its use [P]. Chinese patent: 201110230199, 2011-08-11, 2mmol), 60ml of anhydrous acetic acid, 10ml of concentrated hydrochloric acid in a 250ml round bottom flask, stir in an oil bath at 85°C for 10 minutes, mix well, then drop Add 300 μl of 30% hydrogen peroxide (add in 5 times, drop once every 5 minutes.) Stop the reaction for 20 minutes after the dropwise addition. After cooling, add 250ml of water and immediately find a yellow precipitate. Filter under reduced pressure and wash with water twice. Compound 7 (0.82 g, 90%) was obtained by vacuum filtration and drying.
[0033] Compound 7: 1 H NMR (400 MHz, DMSO) δ 13.83 (s, 1H), 11.67 (s, 1H), 7.45 (s, 1H), 7.16 (s, 1H), 2.42 (s, 3H).
Embodiment 3
[0034] Example 3 2,4,7-trichloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (4),
[0035] 2,4,5,7-Tetrachloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (5) and
[0036] Synthesis of 2,4,5-trichloro-1,3,8-trihydroxy-6-methyl-9,10-anthraquinone (8)
[0037]
[0038] Add 0.54g (2mmol) of emodin, 10ml of hydrochloric acid, 35ml of glacial acetic acid, and 0.82g (8mmol) of manganese dioxide into a 150ml round-bottomed flask in turn, and react at room temperature for about 15 minutes. After the plate reaction is complete, pour it into 200ml of water , precipitated at a high speed, filtered under reduced pressure, washed the filter cake twice with water to remove acetic acid, and separated by column to obtain compounds 4 (456mg, 1.22mmol, 61.0%), 5 (182mg, 0.45mmol, 22%) and 8 (84mg, 0.23mmol, 12%).
[0039] Compound 4: 1 H NMR (400 MHz, DMSO) δ 13.10 (s, 1H), 12.10 (s, 1H), 7.26 (s, 1H), 2.52 (s, 3H).
[0040] Compound 5: 1 H NMR (400MHz, CDCl 3 )δ12.90(s,1H), 12.85(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com