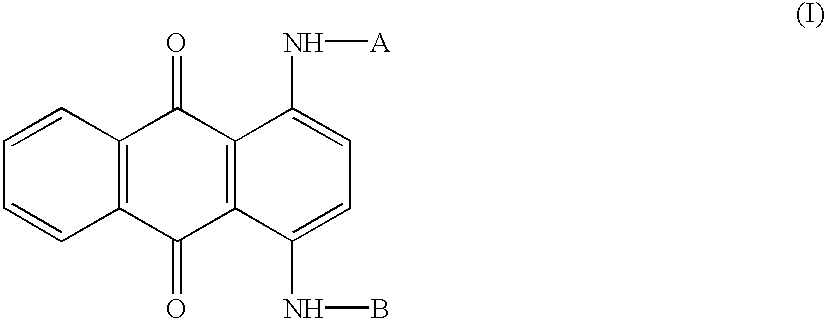
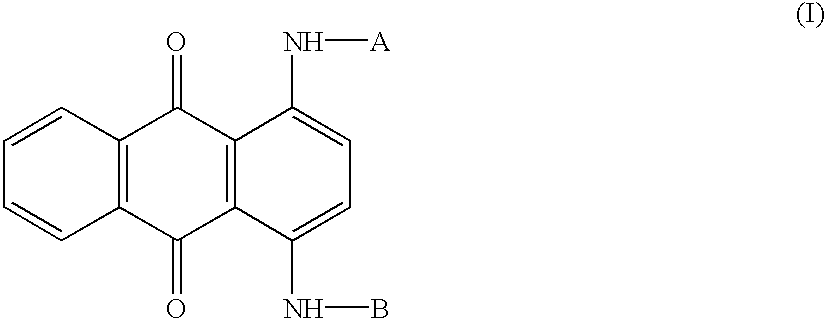
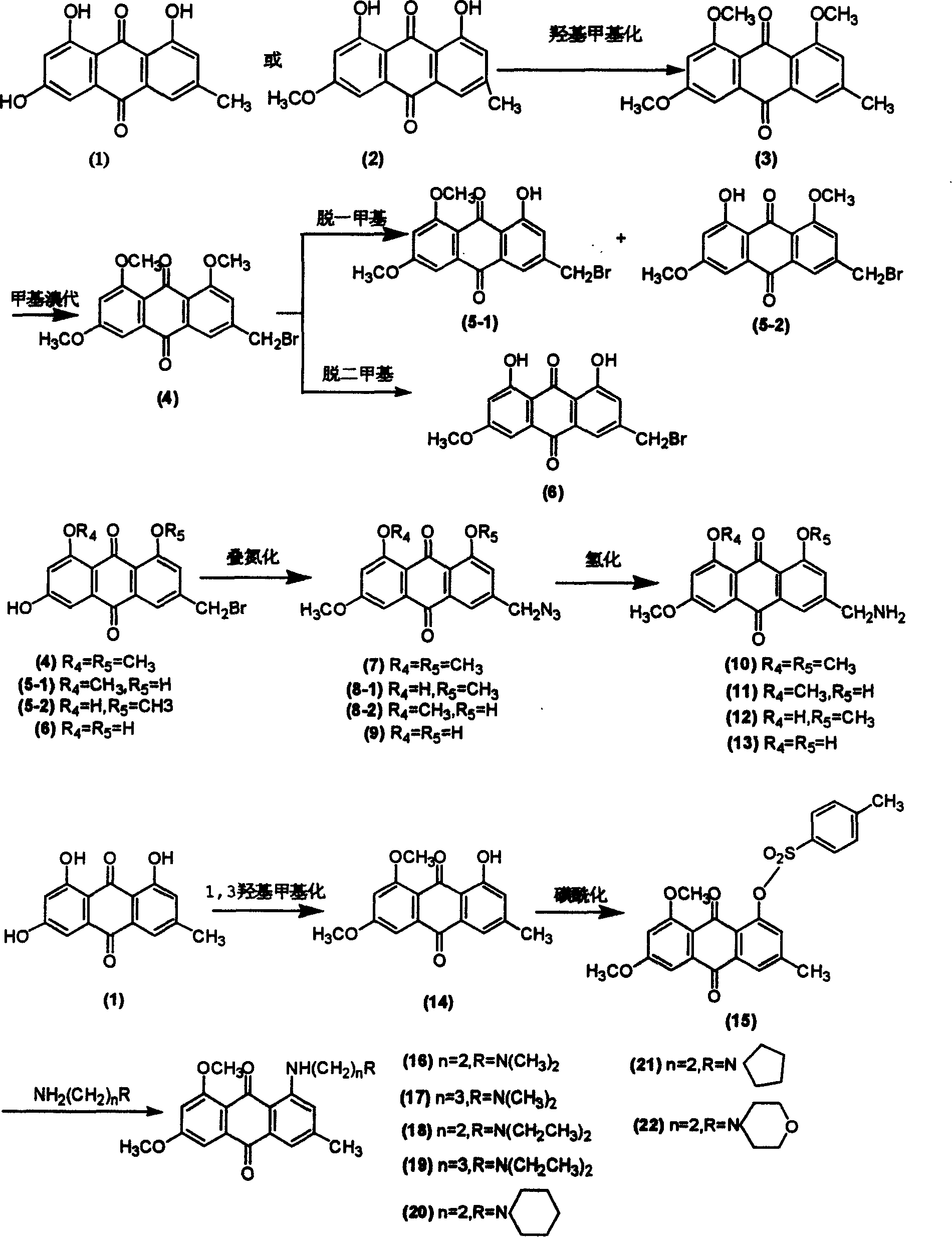
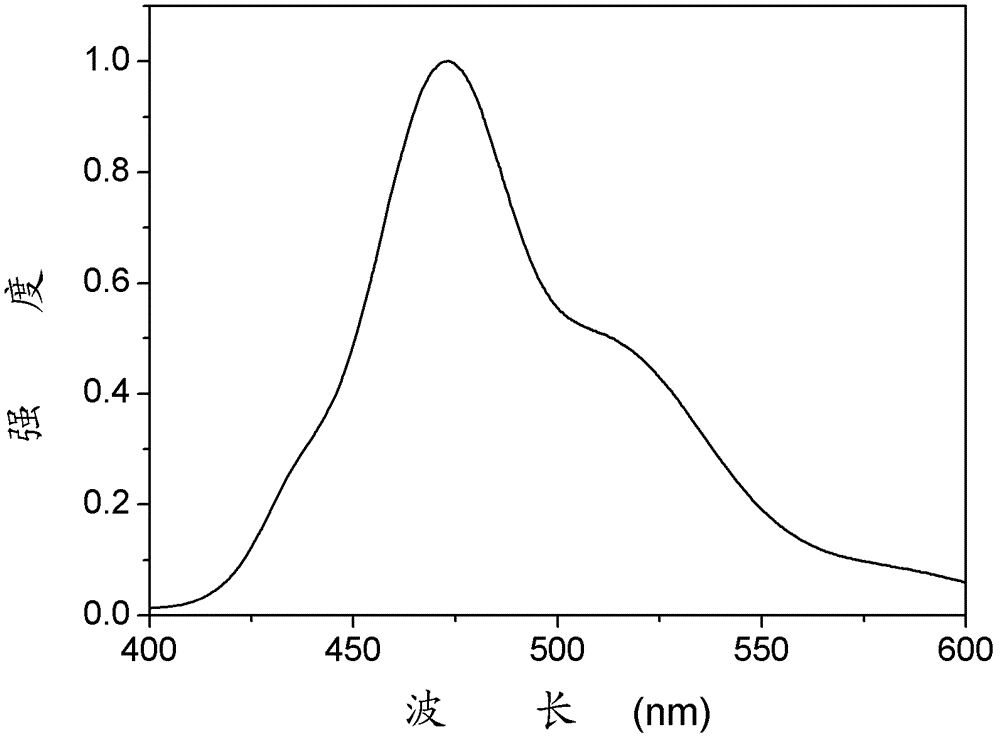
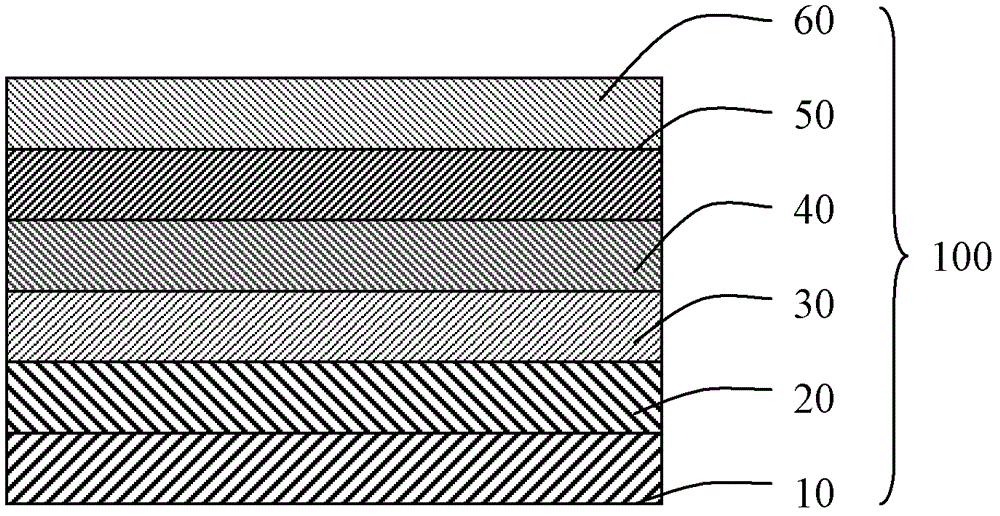
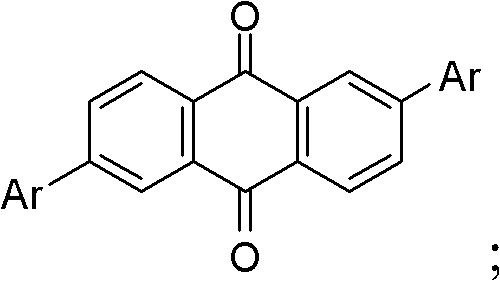
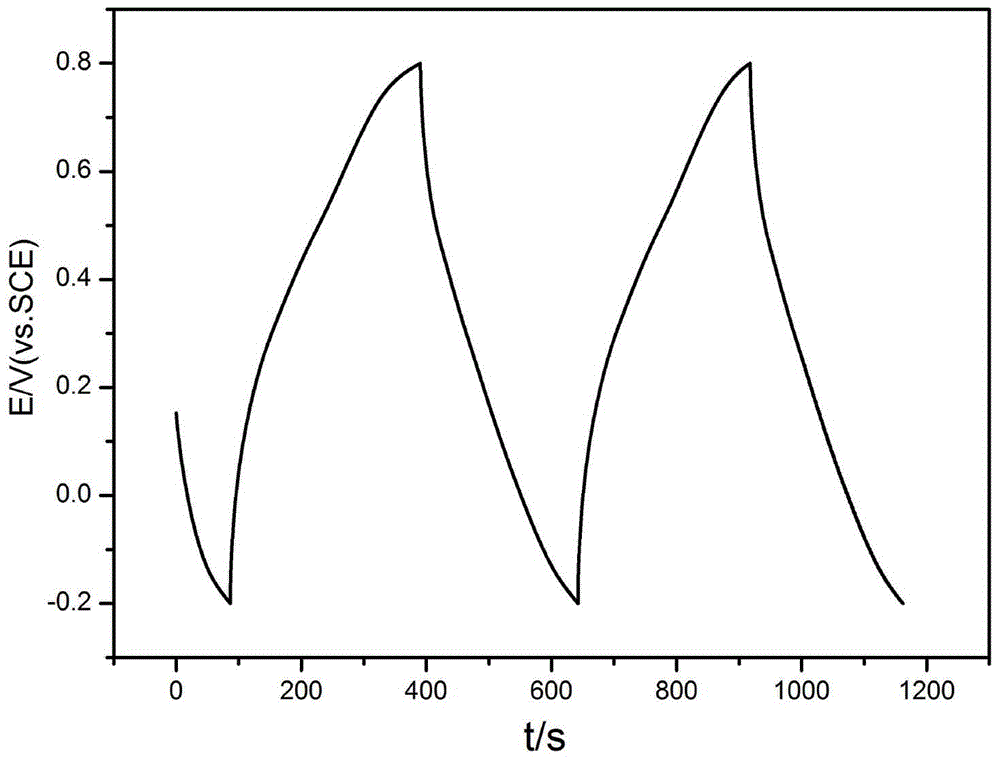
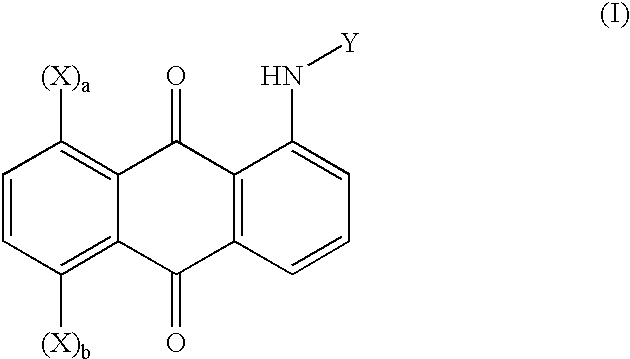
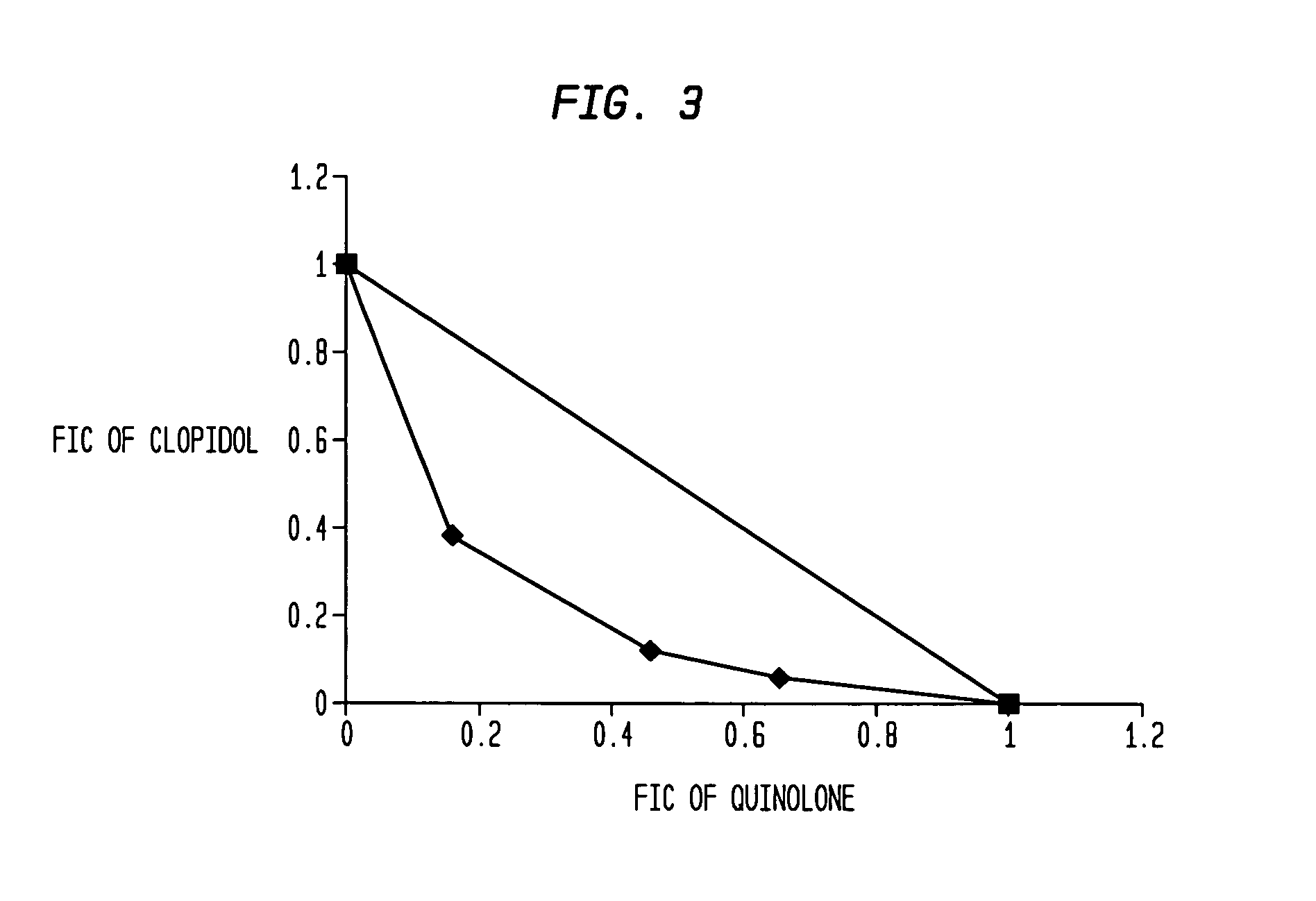
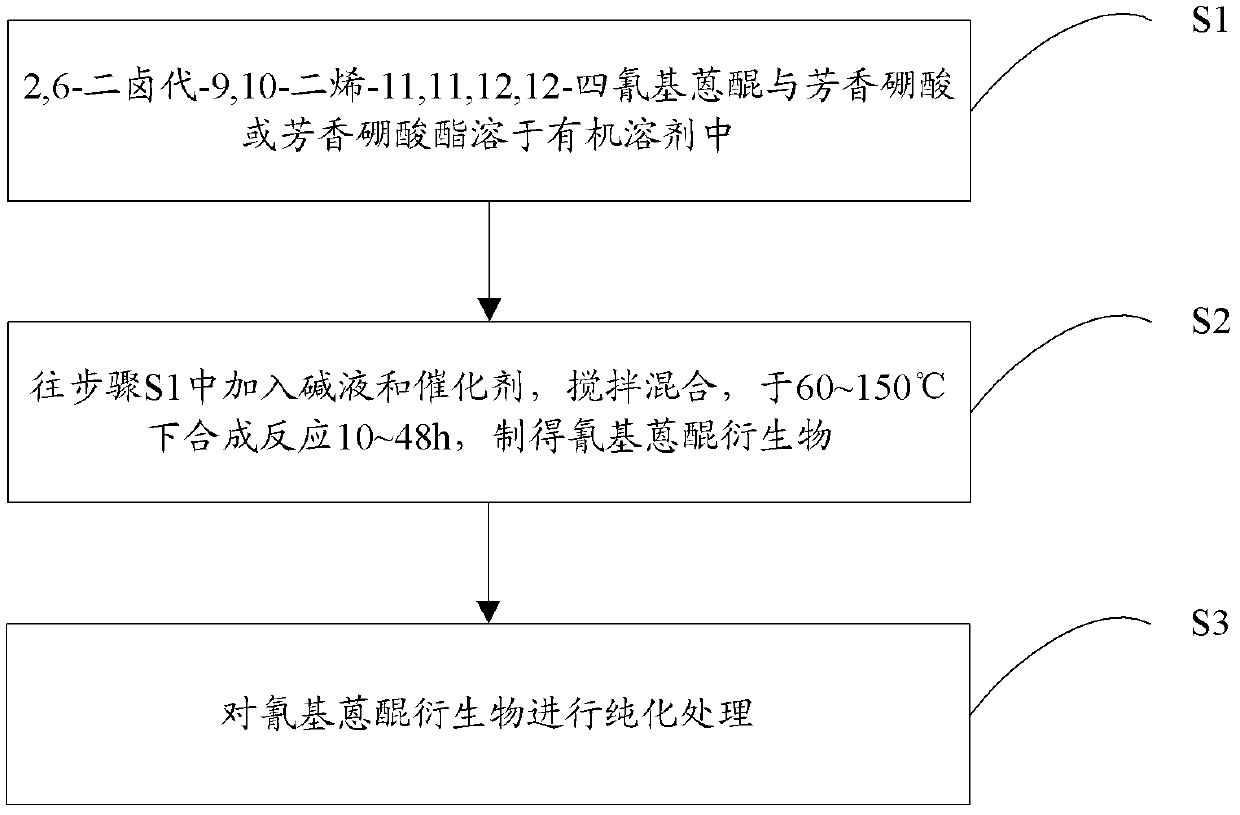
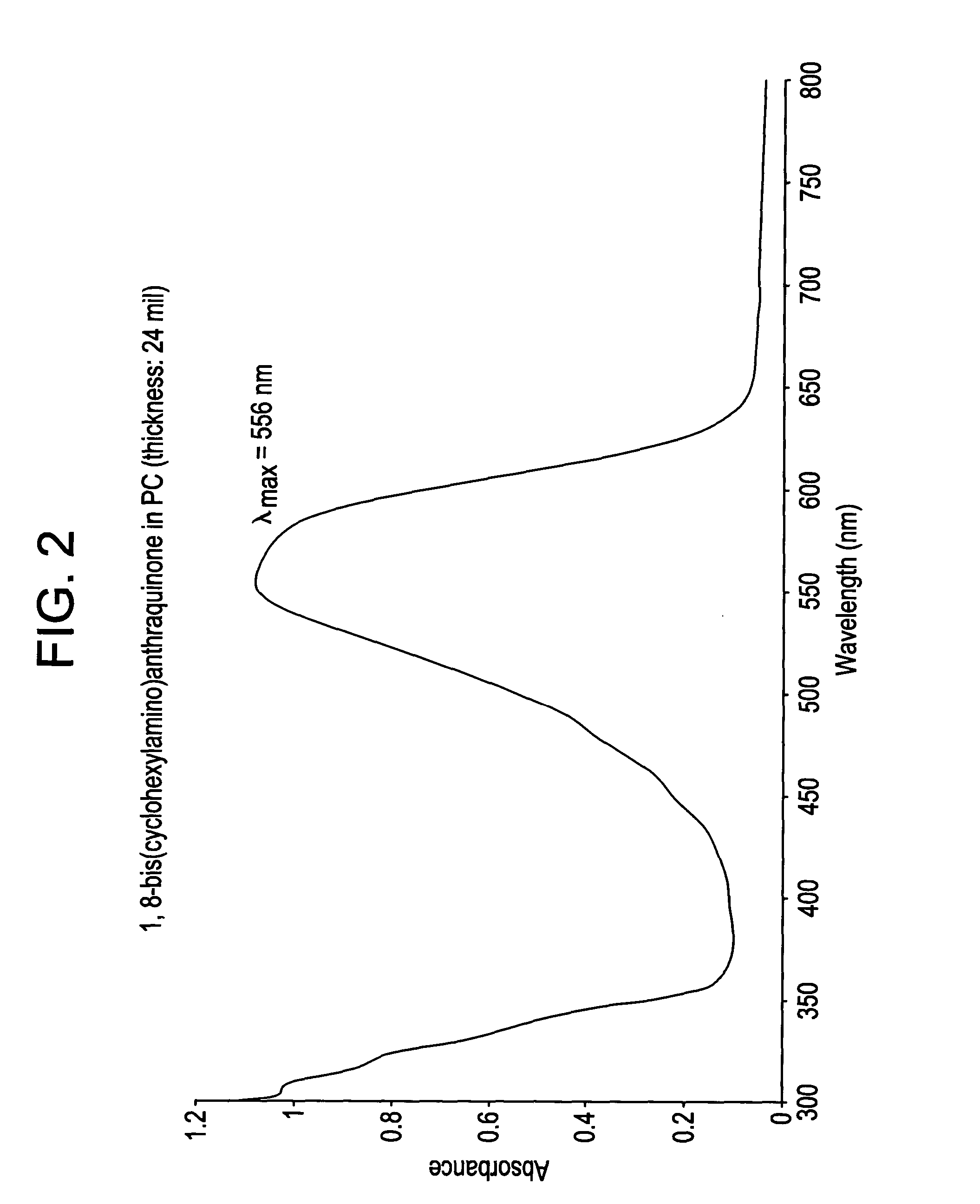
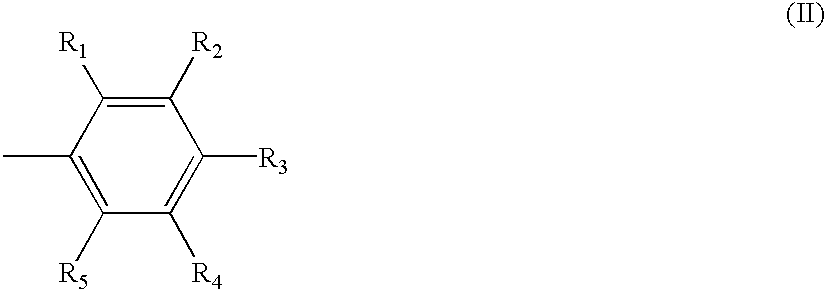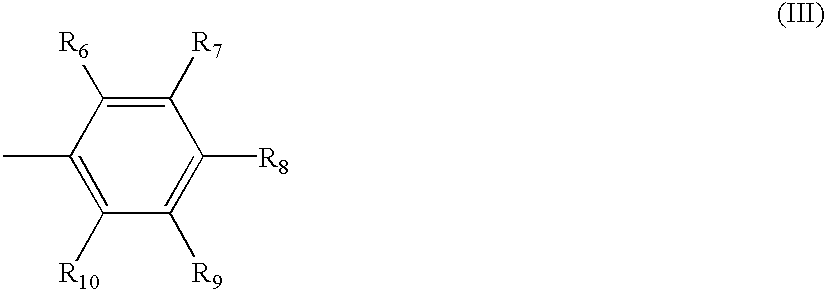Patents
Literature
164 results about "Anthraquinone Derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrogenation method for production of hydrogen peroxide by anthraquinone process
InactiveCN102009960AUniform distributionIncrease contact areaPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesChemical industryHydrogenation process
The invention discloses a hydrogenation method for production of hydrogen peroxide by the anthraquinone process, belonging to the technical field of chemical industry reaction. The method provided by the invention comprises: dispersing the gaseous phase containing hydrogen into the working solution containing the anthraquinone derivatives to obtain the gas-liquid mixing fluid containing micron bubbles, so that the gas-liquid mixing fluid passes through the pipe reactor filled with the hydrogenation catalyst at the space velocity of 3 to 1000h<-1> to finish the hydrogenation process. In the method provided by the invention, the gas-liquid micro-dispersing process and the static bed catalytic hydrogenation process are directly integrated, so that the interphase mass transfer is strengthened, and the contacts between different reactants and between the reactant and the catalyst are more sufficient and more uniform, thereby enhancing the efficiency and the controllability of the hydrogenation process and improving the economical efficiency and the security of the production of the hydrogen peroxide.
Owner:TSINGHUA UNIV
Articles comprising novel polymeric blue anthraquinone-derivative colorants
A blue colorant comprising a chromophore having at least one poly(oxyalkylene) chain attached, through aromatic amino group (or groups), to the 1-position, the 4-position, or both, of an anthraquinone backbone is provided. Such colorants exhibit excellent amine / base stability and thermal stability, effective colorations, excellent low extraction rates, and high lightfastness levels, particularly when incorporated within certain media and / or on the surface of certain substrates, particularly polyesters, polyolefins, and polyurethanes. The poly(oxyalkylene) chain or chains can be conveniently tailored to increase the solubility or compatibility in different solvents or resins thereby permitting the introduction of such excellent coloring chromophores within diverse media and / or or diverse substrates as well as provides a liquid colorant which facilitates handling. Compositions and articles comprising such colorants are provided as well as methods for producing such inventive colorants.
Owner:MILLIKEN & CO
Polymeric blue anthraquinone-derivative colorants
Blue colorants comprising a chromophore having at least one poly(oxyalkylene) chain attached to the 1- position, the 4- position, or both, through aromatic amino group or groups, of an anthraquinone backbone are provided. Such colorants exhibit excellent amine / base stability and thermal stability, effective colorations, excellent low extraction rates, and high lightfastness levels, particularly when incorporated within certain media and / or on the surface of certain substrates, particularly polyesters, polyolefins, and polyurethanes. The poly(oxyalkylene) chain or chains can be conveniently tailored to increase the solubility or compatibility in different solvents or resins thereby permitting the introduction of such excellent coloring chromophores within diverse media and / or or diverse substrates as well as provides a liquid colorant which facilitates handling. Compositions and articles comprising such colorants are provided as well as methods for producing such inventive colorants.
Owner:MILLIKEN & CO
Oxidation method for preparing hydrogen peroxide by anthraquinone method
ActiveCN102009961AUniform distributionIncrease contact areaPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesChemical reactionAnthraquinone Derivatives
The invention discloses an oxidation method for preparing hydrogen peroxide by an anthraquinone method in the technical field of chemical reaction. The method comprises the following steps of: dispersing an oxygen-containing gaseous phase into hydrogenated anthraquinone derivative-containing working solution to prepare a micron-sized bubble-containing gas-liquid mixed fluid, and finishing oxidation reaction when the gas-liquid mixed fluid flows through a delay pipeline. The method can improve the oxidation efficiency of a hydrogenated anthraquinone derivative, shorten oxidation reaction time, reduce the hold-up of the oxidization working solution in a reaction system and improve the safety of the oxidation process.
Owner:TSINGHUA UNIV
Application of antraquinone derivative as pesticide for controlling plant diseases
The present invention relates to an application of anthraquinone derivative as pesticice for controlling plant fungous diseases. The anthraquinone derivative pesticide contains one kind or several kinds of 10 anthraquinone derivative compounds. The anthraquinone derivative compounds have obvious action for preventing and curing several plant diseases, and can be used for preventing and curing economic crop, grain crop, vegetable, fruit tree and melon diseases due to fungi, including sphaerotheca, erysiphe, blumeria, fusarium, sclerotinia, pyricularia, rhizoctonia, colletotrichum, botrytis, verticillium and alternaria.
Owner:INST OF PLANT PROTECTION & SOIL FERTILIZER HUBEI ACAD OF AGRI SCI
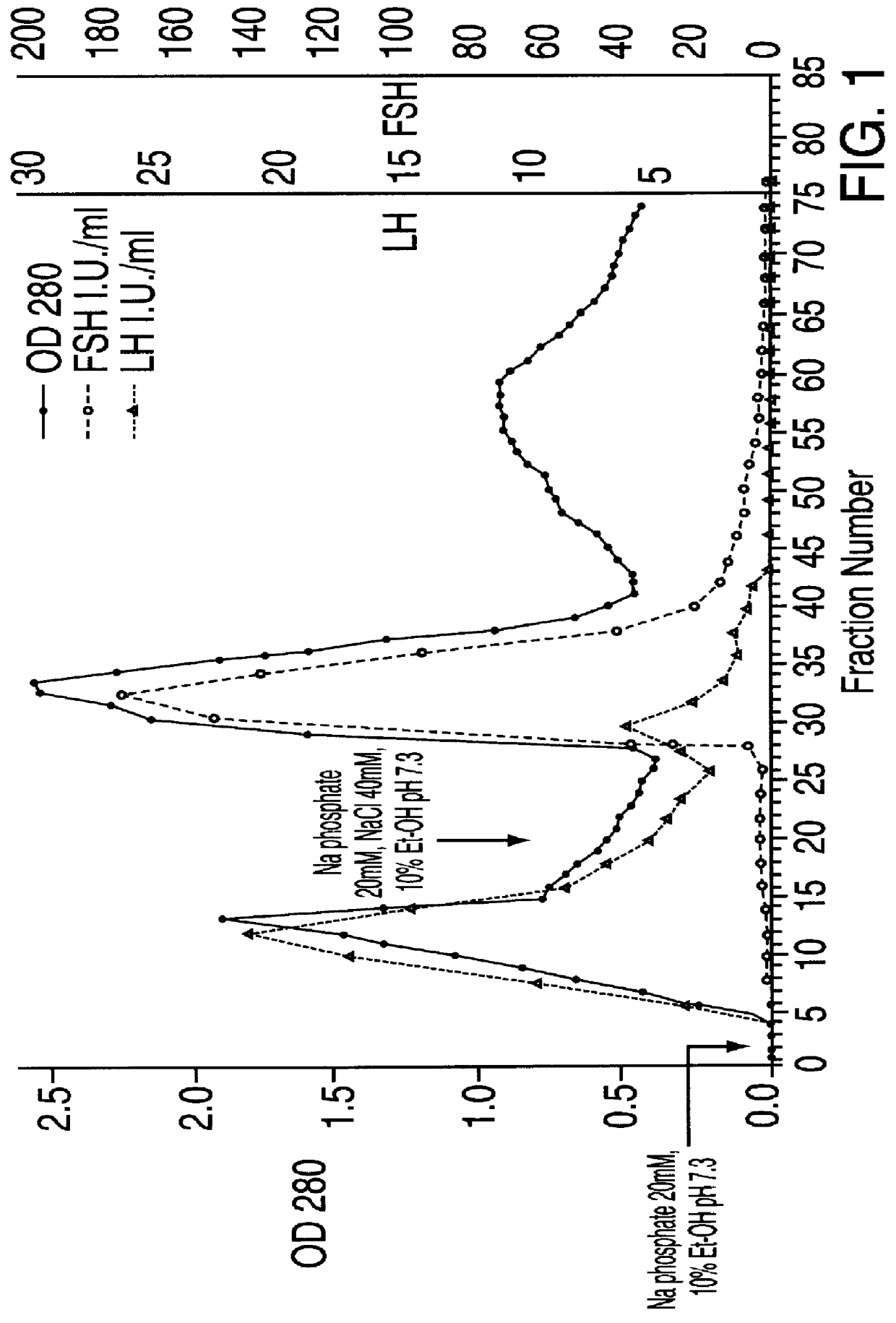
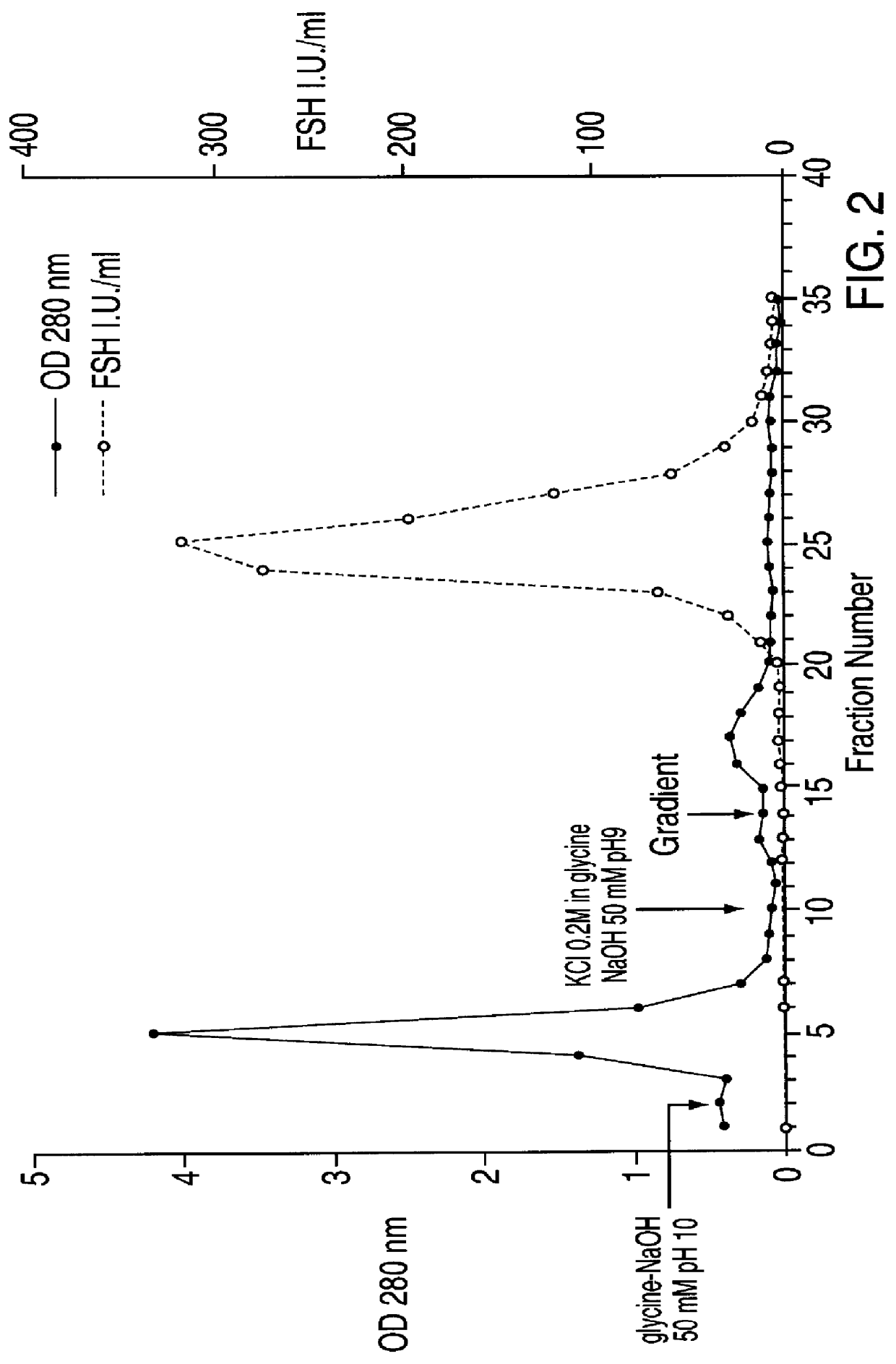
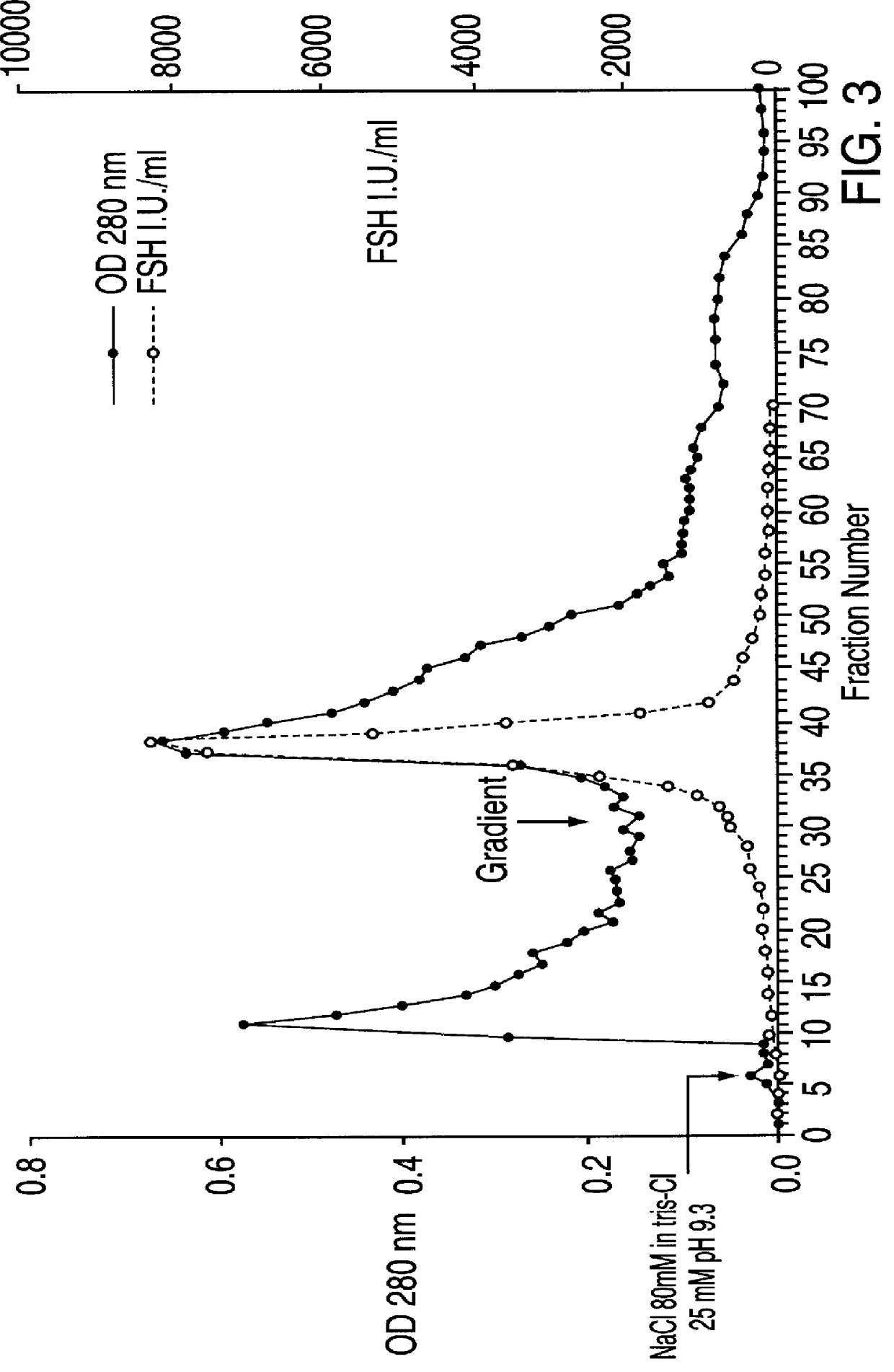
FSH and LH separation and purification process
InactiveUS6162905ASpecific activityHigh purityPeptide/protein ingredientsPeptide preparation methodsDepyrogenationIon exchange
A new process, particularly simple and economical, for FSH and LH separation and purification starting from crude HMG preferably urinary, comprising the following steps: 1) optional exhaustion of crude HMG viral charge in aqueous EtOH 2) ion-exchange chromatography on weakly basic anionic resins of DEAE type; 3) affinity chromatography on resin having an antraquinone derivative as a ligand; 4) optional ion-exchange chromatography on strongly basic anionic resins; Hormones obtained thereby, in particularly pure form and having high specific activity, may subsequently undergo a depyrogenation step.
Owner:ICORE INT +1
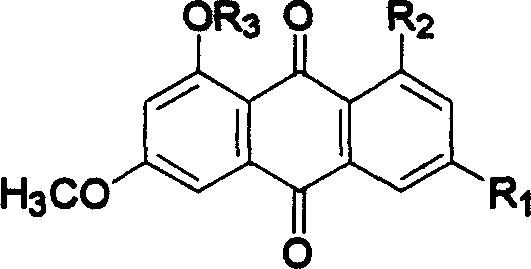
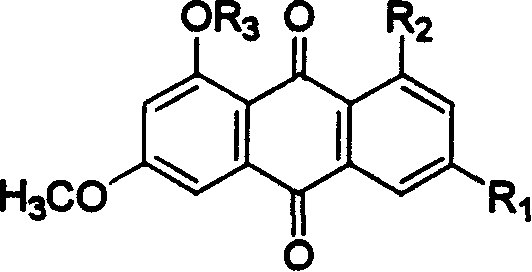
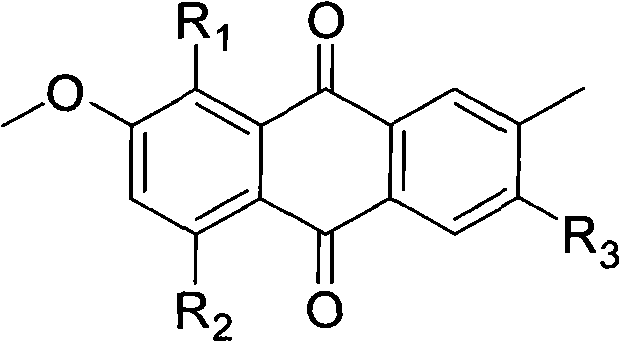
Hydroxyanthraquinone derivatives and their application in preparation of anticancer medicines
InactiveCN1546451AGood inhibitory effectLow toxicityOrganic active ingredientsOrganic chemistryHydroxyanthraquinoneAnthraquinone Derivatives
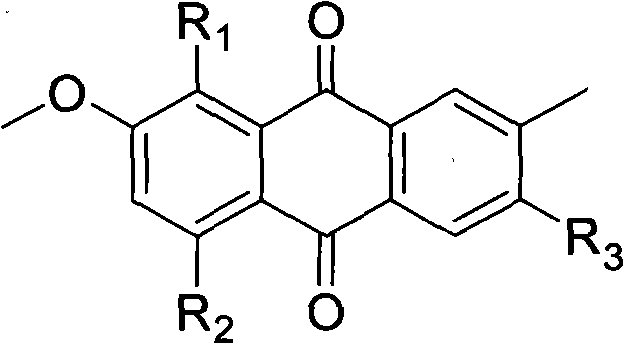
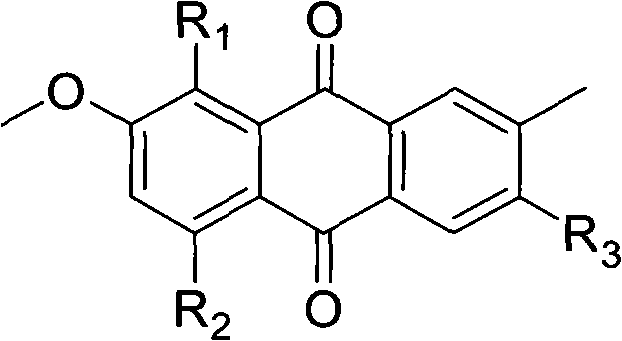
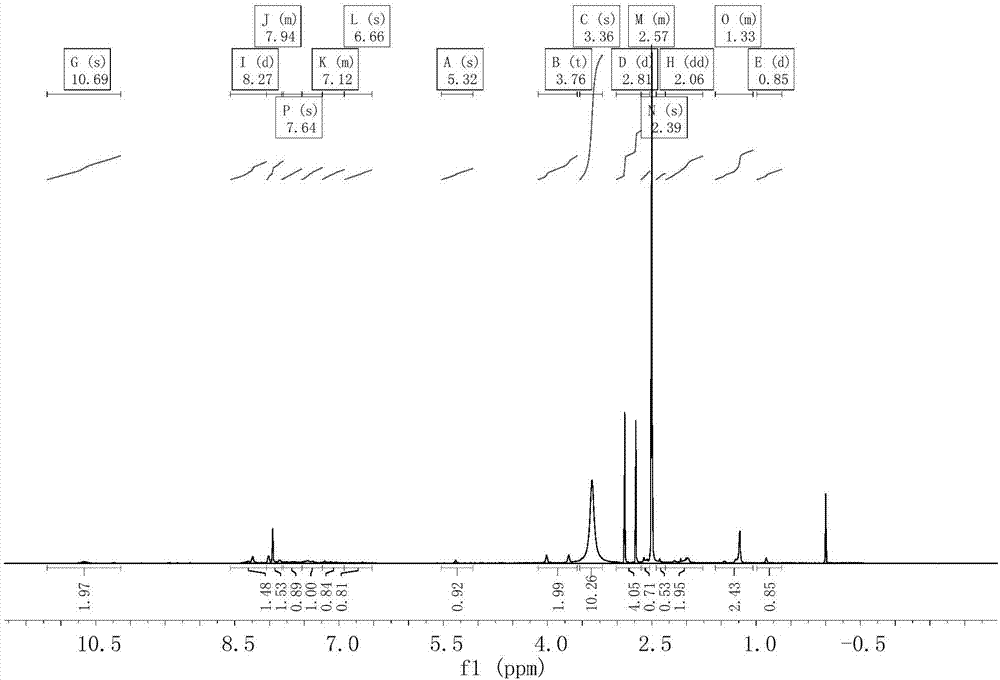
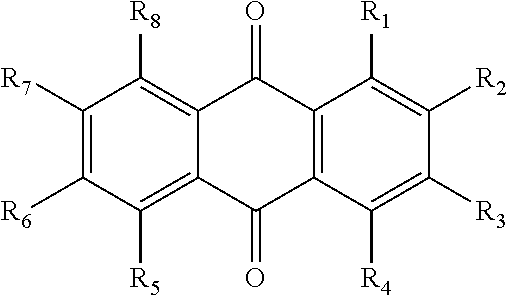
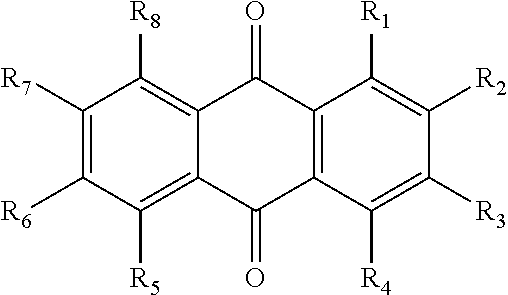
The invention relates to hydroxy anthraquinone derivatives and their use in preparing anti-cancer medicament, test has shown that the hydroxy anthraquinone derivatives have appreciable inhibitory action for various tumor cell strains, thus can be used in preparing cancer treatment medicament. The chemical structural formula of the hydroxy anthraquinone derivatives is shown by I, wherein R1, R2,R3 are defined in the description.
Owner:SUN YAT SEN UNIV
Light-cured three dimensional printing material and preparation method thereof
The invention discloses a light-cured three dimensional printing material and a preparation method thereof. The light-cured three dimensional printing material comprises, by mass, 40-45 parts of polyester modified epoxy acrylate, 8-12 parts of cycloaliphatic epoxy resin, 8-10 parts of trihydroxymethylpropyl trimethylacrylate, 16-20 parts of 1,6-hexanediol diacrylate, 2-8 parts of triethyleneglycol divinyl ether, 1.5-2 parts of isopropylthioxanthone, 1.5-2 parts of 2-phenyl-2,2-dimethylamino-1-(4-morpholinylphenyl)-1-butanone, 1.5-2 parts of a reactive tertiary amine co-initiator, 0.5-2.7 parts of 4-isobutylphenyl-4'-methylphenyliodonium hexafluorophosphate, 0.5-2 parts of an anthraquinone derivative, 0.5-2 parts of a promoter and 4-8 parts of a flexibilizer. Shaped parts made by using the light-cured three dimensional printing material have the advantages of low shrinkage, high toughness and high shaping precision.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS +2
Coloring composition for color filter and color filter
A color filter has a filter segment. The filter segment is formed by means of a color composition for color filter. The color composition for color filter includes: a phosphate-ester series pigment dispersant; a pigment derivatives having a certain alkali-base; at least one alkali-base-containing derivatives selected from pigment derivatives, a Anthraquinone derivatives and a triazine derivatives; a pigment carrier; and a pigment.
Owner:TOYO INK SC HOLD CO LTD
Anthraquinone derivative material and preparation method and application thereof
ActiveCN102795983AImprove thermal stabilityImprove structural stabilityOrganic compound preparationSolid-state devicesAnthraquinone DerivativesThermal stability
The invention discloses an anthraquinone derivative material, which has the following structural formula: wherein -Ar is FORMULA. The anthraquinone derivative material has excellent thermal stability and conjugate structure, and emits blue light. The invention also provides a preparation method for the anthraquinone derivative material and application of the material in organic electroluminescent devices.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Application of compound to preparation of medicines for treating viral pneumonia
ActiveCN111228275AExcellent antiviral pneumonia effectStrong anti-virusAntiviralsRespiratory disorderPurinePharmacology
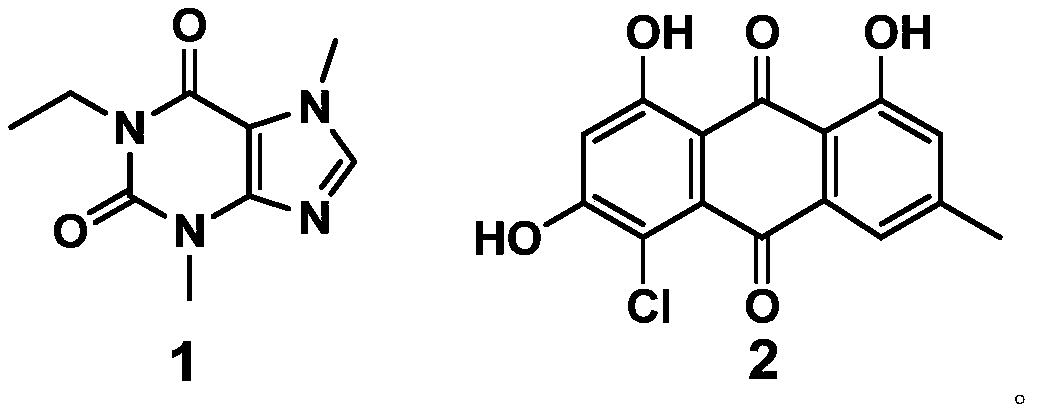
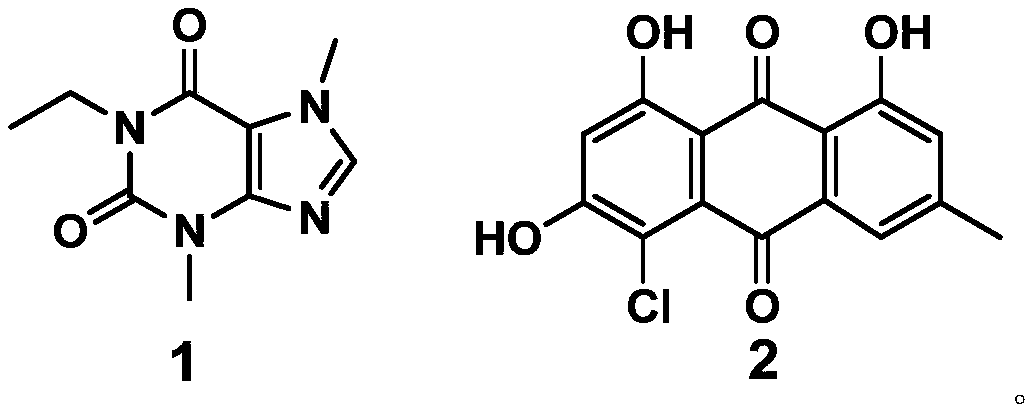
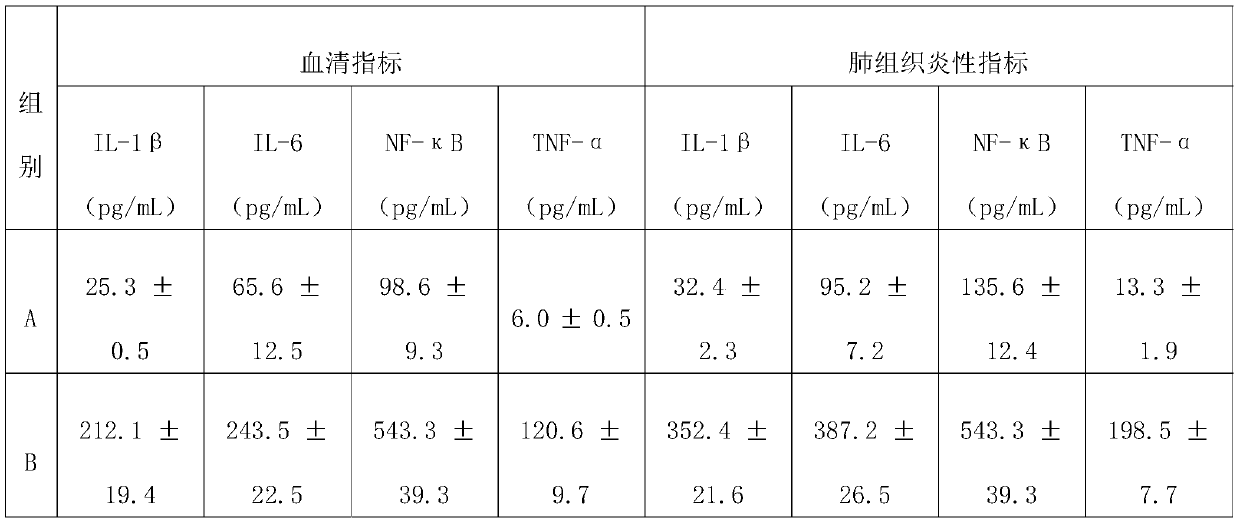
The invention discloses an application of a compound to preparation of medicines for treating viral pneumonia, and relates to the technical field of drugs. The technical problem that novel antiviral drugs are deficient in the prior art, can be solved. The compound comprises a purine compound adopting the structure as shown in the formula 1, and / or an anthraquinone derivative adopting the structureas shown in the formula 2. The purine compound adopting the structure as shown in the formula 1, and / or the anthraquinone derivative adopting the structure as shown in the formula 2 both can show predominant effect for resisting viral pneumonia when being separately used or used jointly. Drugs for treating viral pneumonia, are prepared by the compound provided by the invention are more notable inactivity compared with clinical frontline drugs, and are better than drugs of ribavirin, oseltamivir and the like.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Preparation method of anthraquinone-molecule non-covalent modified graphene/conductive polymer composite
InactiveCN104867702AGuaranteed conductivityEnhanced ion/electron transfer capabilityHybrid capacitor electrodesHybrid/EDL manufactureAnthraquinonesConductive polymer composite
The invention discloses a preparation method of an anthraquinone-molecule non-covalent modified graphene / conductive polymer composite. The method comprises the steps of dissolving an anthraquinone derivative in a graphene oxide solution, performing stirring and ultrasonic dispersion, adding a conductive polymer monomer to the solution, performing stirring, ultrasonic dispersion and uniform mixing, and performing stirring and a reaction under a heating condition to prepare the anthraquinone-molecule non-covalent modified graphene / conductive polymer composite. According to the method, an anthraquinone molecule is anchored on the surface of graphene oxide by utilizing hydrogen-bond interaction and pi-pi interaction between the anthraquinone molecule and graphene oxide, an oxygen-containing functional group on the surface of graphene oxide serves as an oxidant, the monomer is oxidized into a conductive polymer, and graphene oxide is reduced into graphene, so that the prepared composite represents high conductivity and stability and can serve as a potential electrode material of a supercapacitor.
Owner:HOHAI UNIV
Polymeric 1,5- or 1,8-disubstituted anthraquinone-derivative colorants and articles comprising such colorants
Red colorants comprising a chromophore having at least one poly(oxyalkylene) chain attached to the 1-position, as well as at least one poly(oxyalkylene) chain attached to either the 5-position or the 8-position of an anthraquinone backbone are provided. Such colorants exhibit excellent base stability, effective colorations, excellent low extraction rates, and high lightfastness levels, particularly when incorporated within certain media and / or on the surface of certain substrates, particularly polyesters. The poly(oxyalkylene) chains also increase the solubility in different solvents or resins thereby permitting the introduction of such excellent coloring chromophores within diverse media and / or diverse substrates as well as provides a liquid colorant which facilitates handling. Compositions and articles comprising such colorants are provided as well as methods for producing such inventive colorants are also provided.
Owner:MILLIKEN & CO
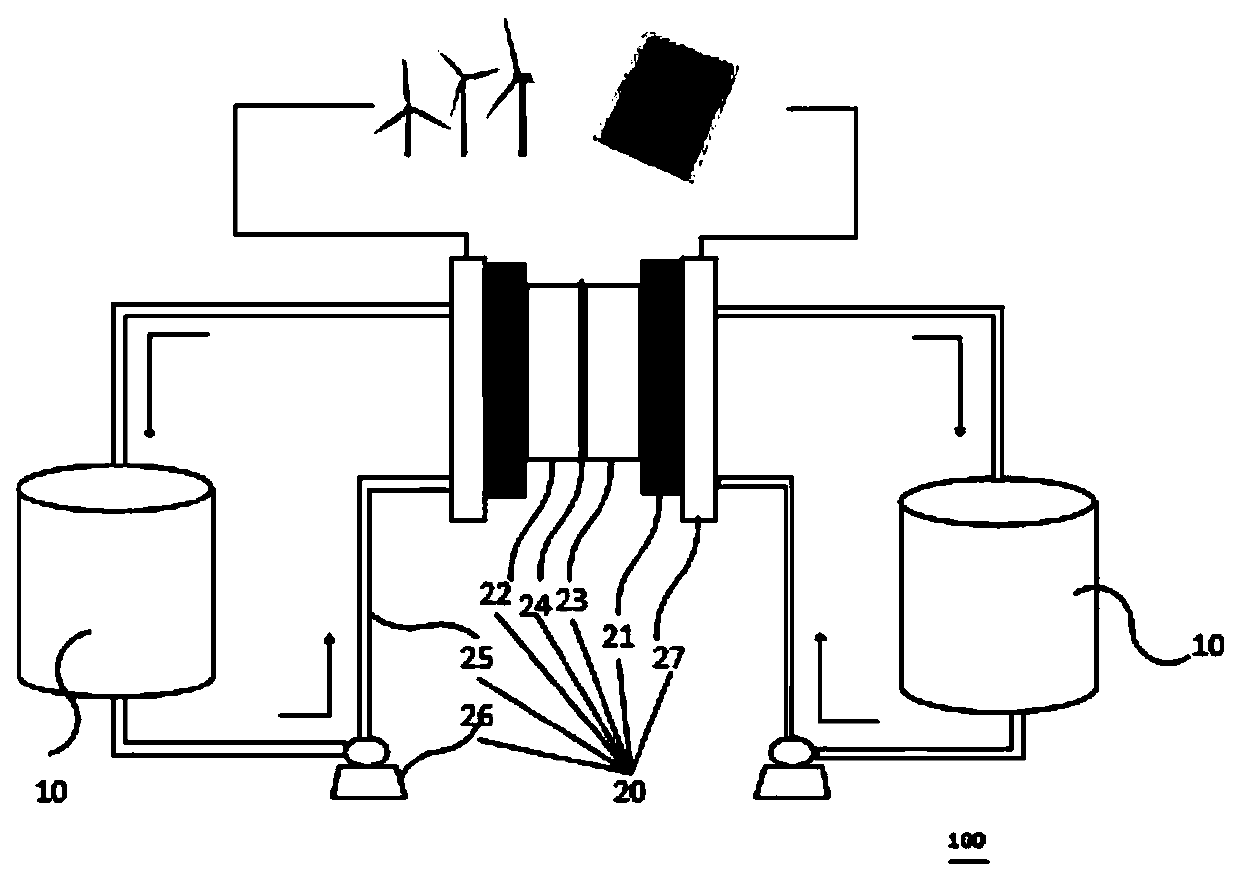
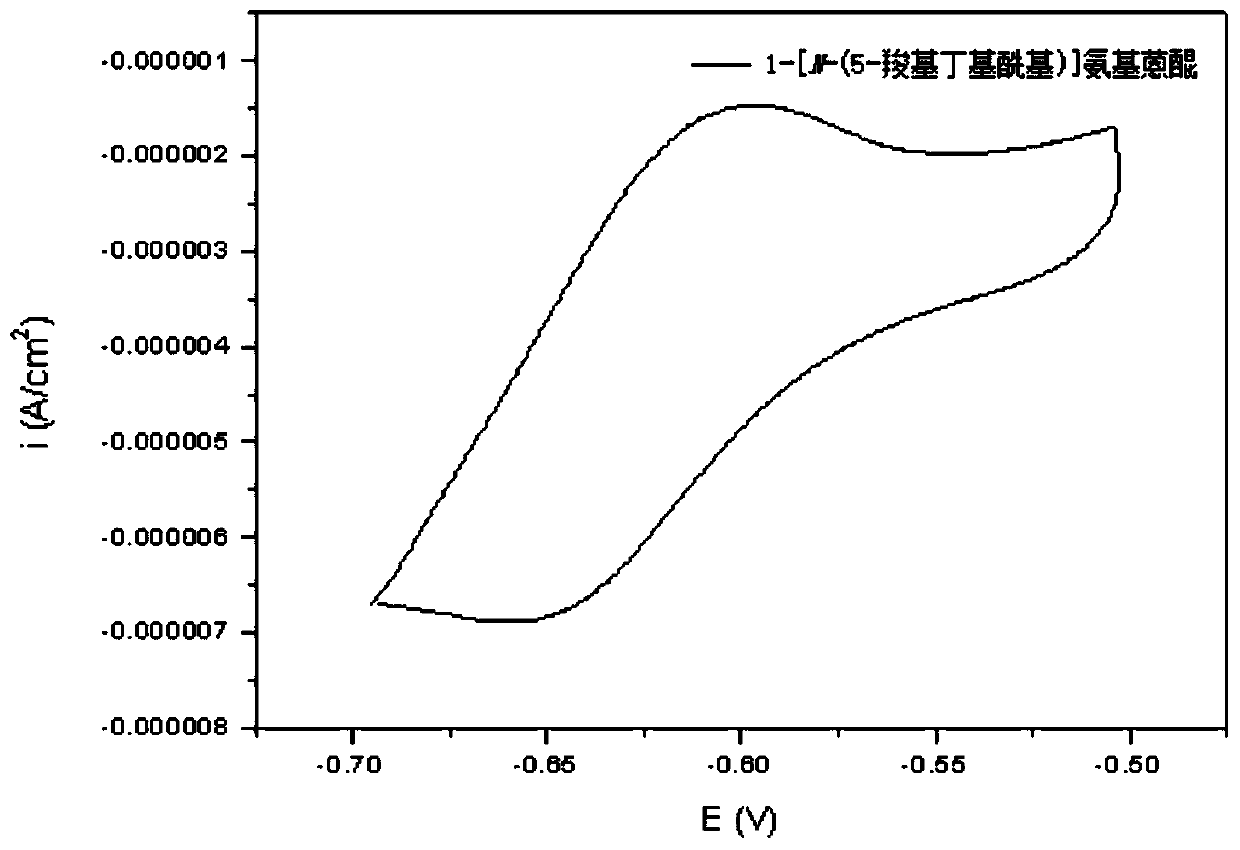
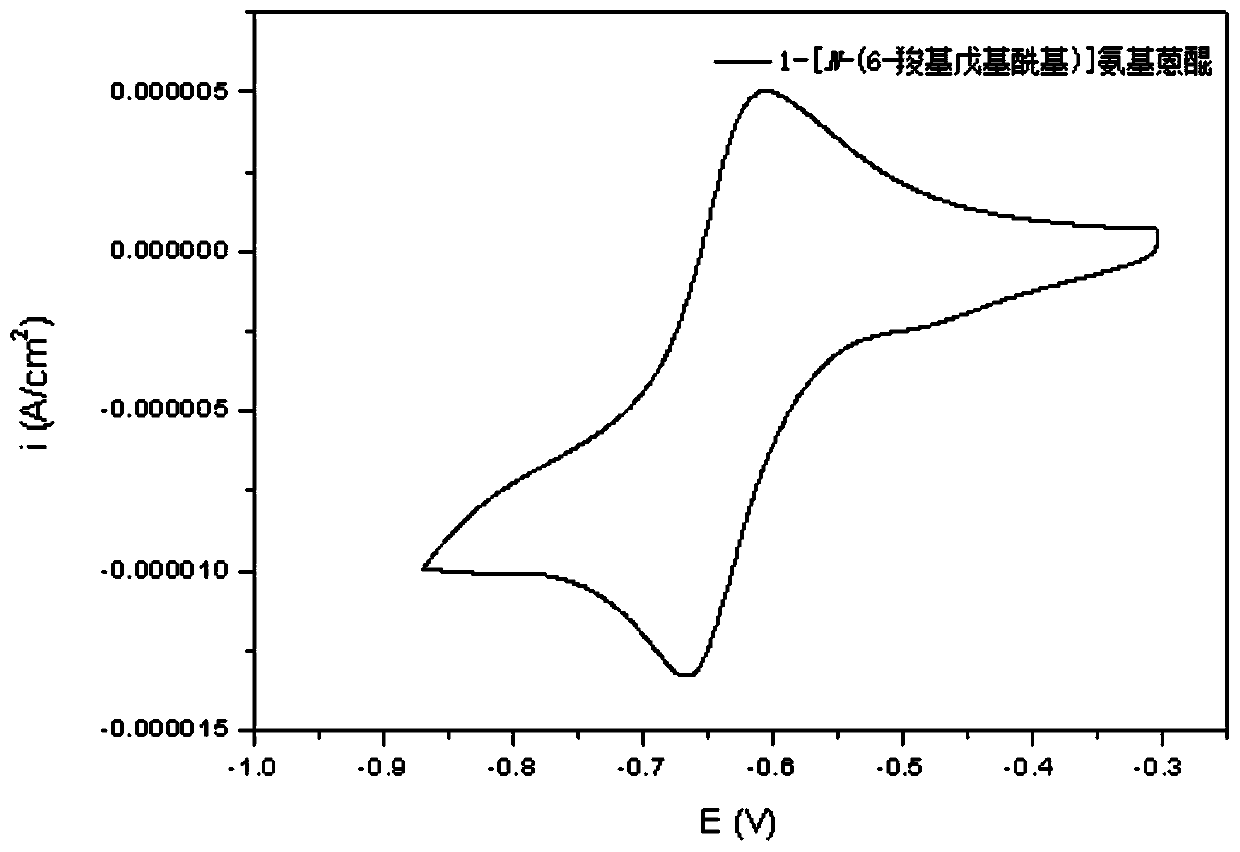
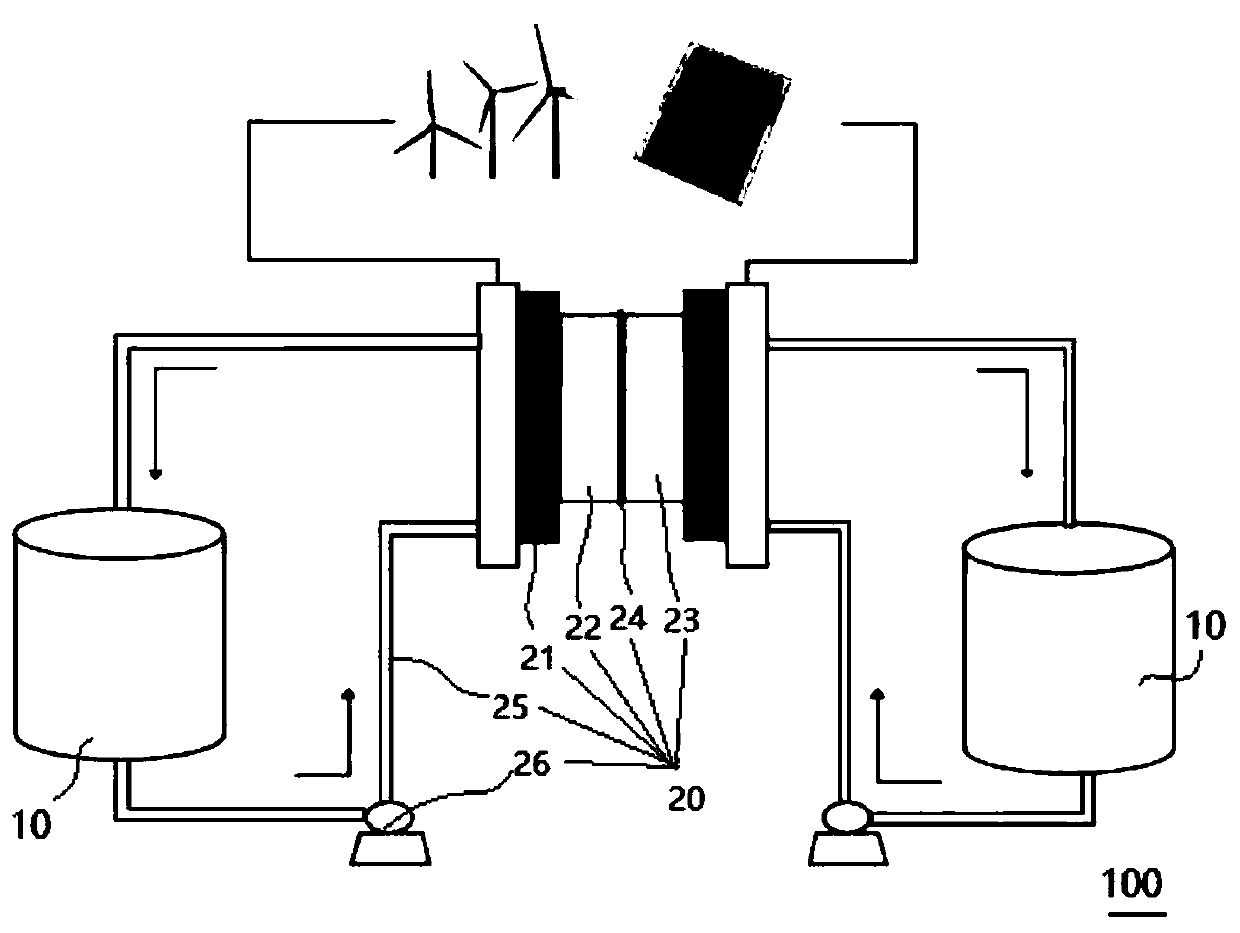
Redox flow battery system based on amino-anthraquinone derivative
InactiveCN110444787ALow costImprove securityElectrolyte stream managementRegenerative fuel cellsSupporting electrolyteSolubility
The invention provides a redox flow battery (RFB) system based on an amino-anthraquinone derivative. The RFB system comprises two electrolyte reservoirs spaced apart from each other, wherein an electrolyte is stored in a tank or a solution chamber and includes a positive electrode active material, a negative electrode active material and a supporting electrolyte, the positive electrode active material is a 2,2,6,6-tetramethylpiperidine oxynitride (TEMPO) compound, the negative electrode active material is an anthraquinone derivative containing a carboxyl group, the positive electrode active material and the negative electrode active material are directly dissolved or dispersed in a bulk form in a system using water as a solvent and are stored in two salt caverns respectively, and the supporting electrolyte is dissolved in the system; and a RFB stack communicating with the two electrolyte reservoirs. The RFB system based on the amino-anthraquinone derivative according to the embodimentof the present invention has the advantages of low cost, high safety performance, stable charge-discharge performance, and high active material solubility.
Owner:CHINASALT JINTAN
Anthraquinone derivative, as well as preparation method and application of anthraquinone derivative serving as antibacterial agent
InactiveCN102603525AHas selective inhibitory activityAntibacterial agentsOrganic active ingredientsEscherichia coliBacillus cereus
The invention provides an anthraquinone derivative, as well as a preparation method and application of the anthraquinone derivative serving as an antibacterial agent. The preparation method comprises the following steps: culturing a strain of Nigrospora sp. (HM565952) in a strain culture medium; fermenting the nigrospora sp. in a fermentation culture medium; filtering the fermentation broth, removing thallus, and extracting the fermentation broth with ethyl acetate; concentrating the extract, and performing chromatographic separation, and concentrating the eluent to obtain two types of yellow powder, namely 3,5,8-Trihydroxy-7-methoxy-2-methylanthracene-9,10-dione and austrocortirubin; and adding an acetic anhydride reagent in a solution dissolved with the compound, and reacting to obtain the anthraquinone derivative. The anthraquinone derivative obtained from the Nigrospora sp. (HM565952) has selective inhibitory activity to Bacillus subtilis, Bacillus cereus, Micrococcus tetragenus, Micrococcus lutea, Escherichia coli, Staphylococcus aureus, Staphylococcus albus, Vibrio parahaemolyticus and Vibrio anguillarum, and can be used for developing selective bacteriostatic agents; and the raw materials can be produced on a large scale, and not limited by resources. Therefore, the anthraquinone derivative has the advantage of wide application prospect.
Owner:OCEAN UNIV OF CHINA
Hydracid bisazo double-active-group reactive brilliant blue dyes, and preparation method and application thereof
InactiveCN106905718AColorfulSimple preparation processReactive dyesDyeing processCellulose fiberAnthraquinone Derivatives
The invention relates to hydracid bisazo double-active-group reactive brilliant blue dyes, and a preparation method and application thereof. The structural formula of the dyes is disclosed in the specification, wherein when R1=-SO3Na or H, R2=-H; and when R2=-COONa, R1=-H, and R3 is disclosed in the specification. The preparation method comprises the following steps: carrying out reaction on cyanuric chloride and 3,5-diamido-2,4,6-trimethylbenzenesulfoamino substituted anthraquinone derivatives to obtain a primary polycondensation solution; adding 2,4-diamidobenzenesulfonic acid (sodium 2,4-diamidobenzenesulfonate) or 3,5-diamidobenzoic acid (sodium 3,5-diamidobenzoate), and reacting to obtain a secondary polycondensation substance; carrying out diazotization on the para-position ester or meta-position ester to obtain diazonium salts; adding hydracid, and reacting to obtain an acidic coupling product; carrying out diazotization on the secondary polycondensation substance to obtain secondary polycondensation substance diazonium salts; and adding the secondary polycondensation substance diazonium salts into the acidic coupling product, and reacting to obtain the hydracid bisazo double-active-group reactive brilliant blue dyes. The dyes have the advantages of bright color, high fixation rate, favorable fastness, short dyeing equilibrium time and high substantivity, and can be used for dyeing and dye printing of cellulose fibers and protein fibers.
Owner:TAIZHOU QIANJIN CHEM CO LTD
Application of emodin anthraquinone derivative in preparation of anti-hepatocellular carcinoma drugs
InactiveCN103356515AAvoid damageObvious cytotoxic effectOrganic active ingredientsAntineoplastic agentsApoptosisCancer cell proliferation
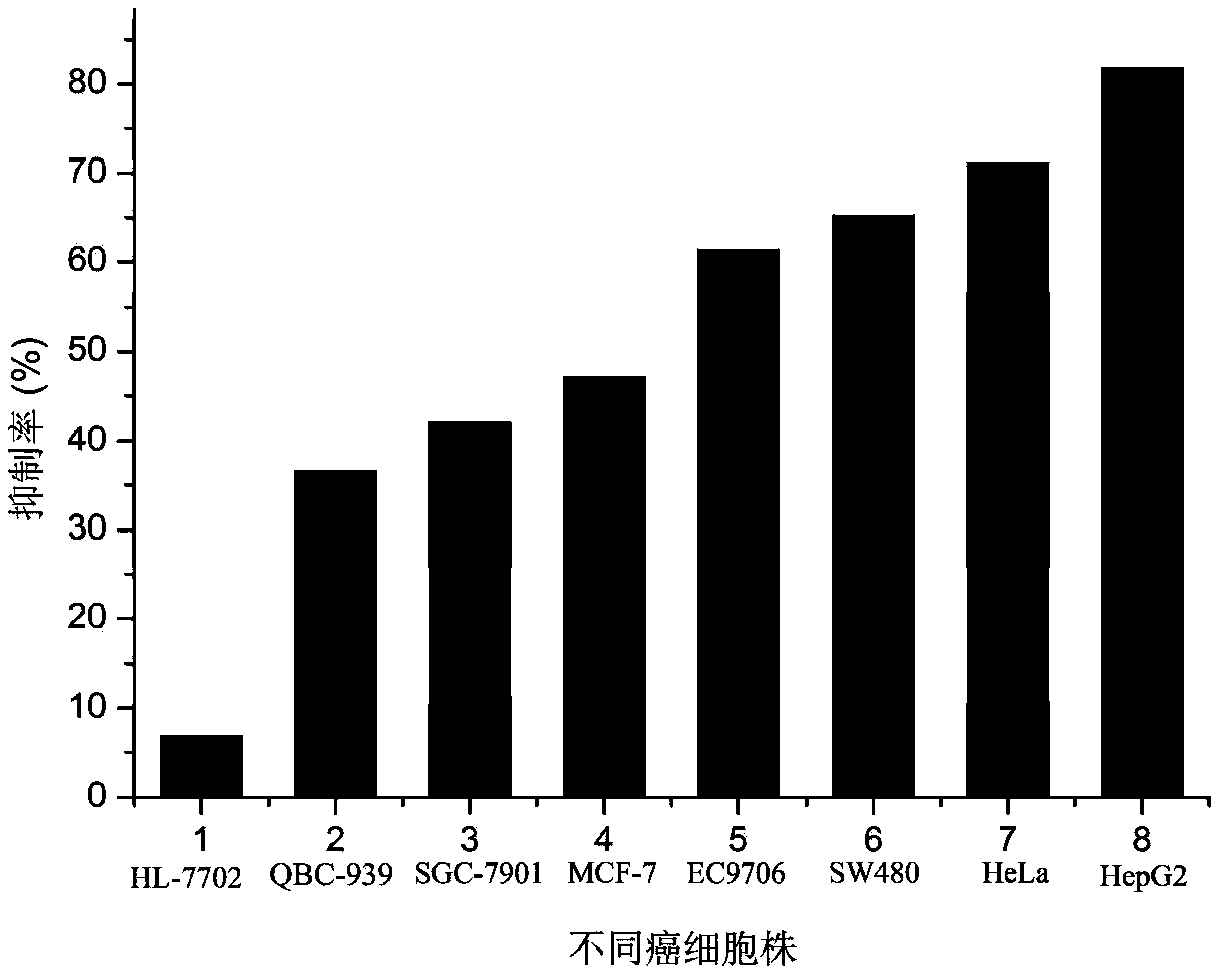
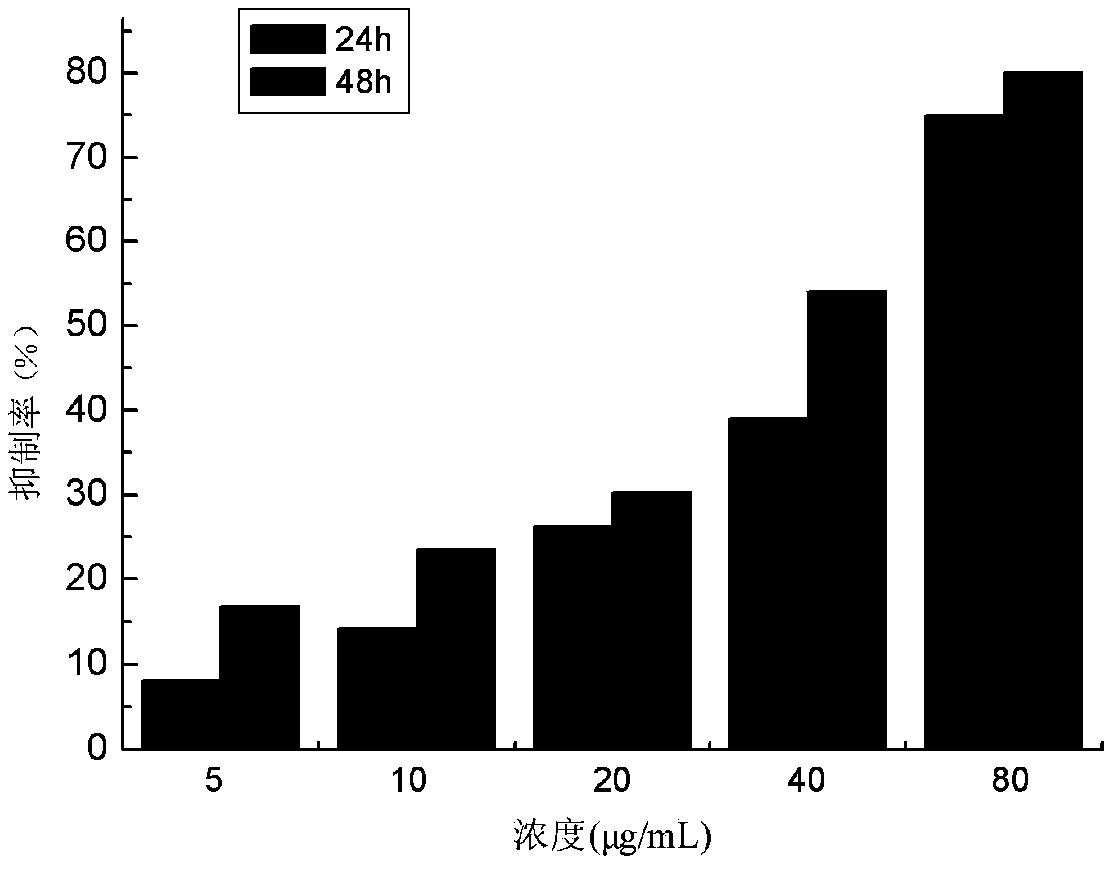
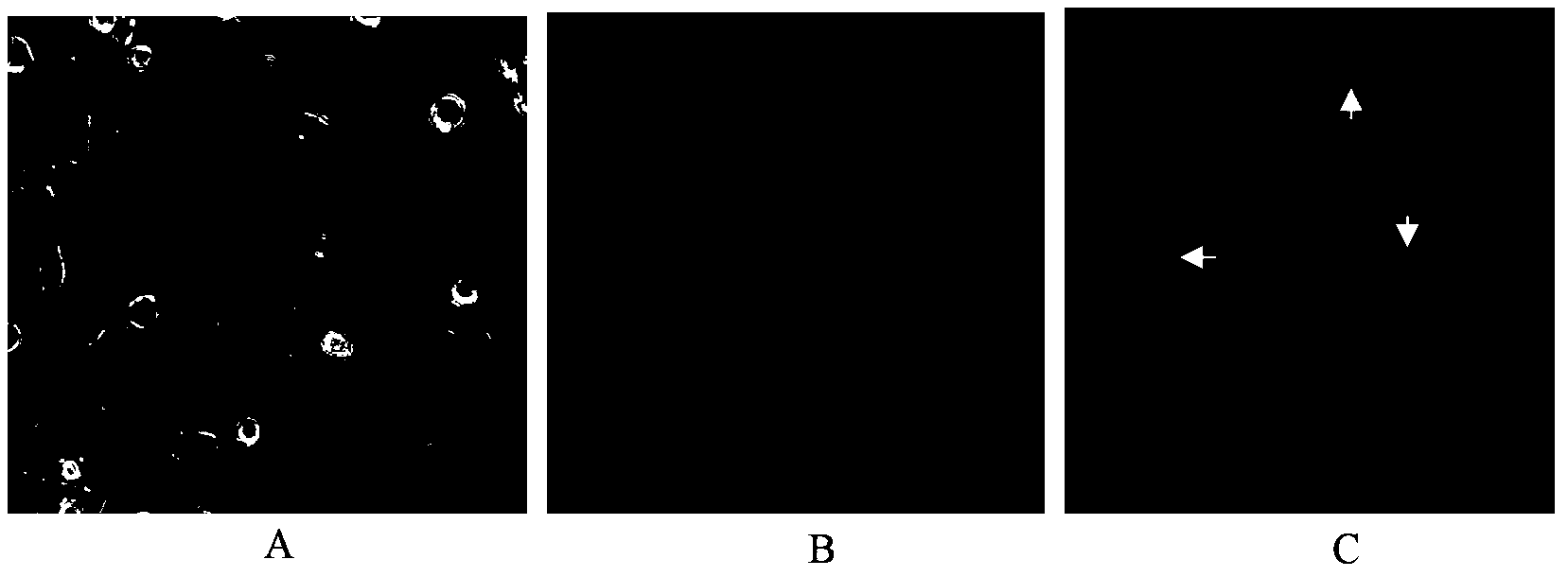
The invention provides application of an emodin anthraquinone derivative in preparation of anti-hepatocellular carcinoma drugs. The emodin anthraquinone derivative is a 1, 4-dimethyl-6, 8-dimethoxy-9, 10-anthraquinone. The derivative not only can well inhibit proliferation of hepatocellular carcinoma cells, induce their apoptosis, and also has very small toxicity to normal cells. The derivative can be used in conjunction with 5-FU, cis-platinum and other chemotherapy drugs to increase the sensitivity of the chemotherapy drugs, thus achieving the effect of inhibiting hepatocellular carcinoma cell growth. Therefore, the emodin anthraquinone derivative can be used for preparing anti-hepatocellular carcinoma drugs or adjuvant chemotherapy drugs.
Owner:SHANXI UNIV
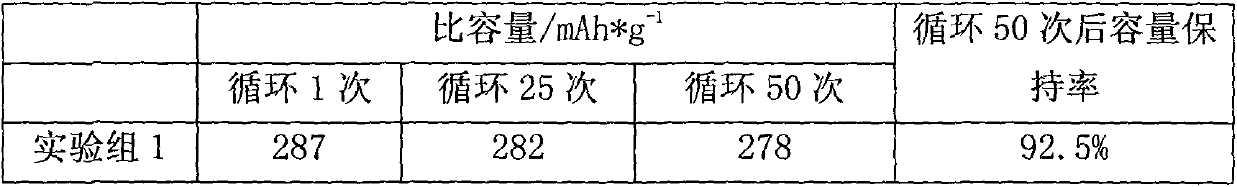
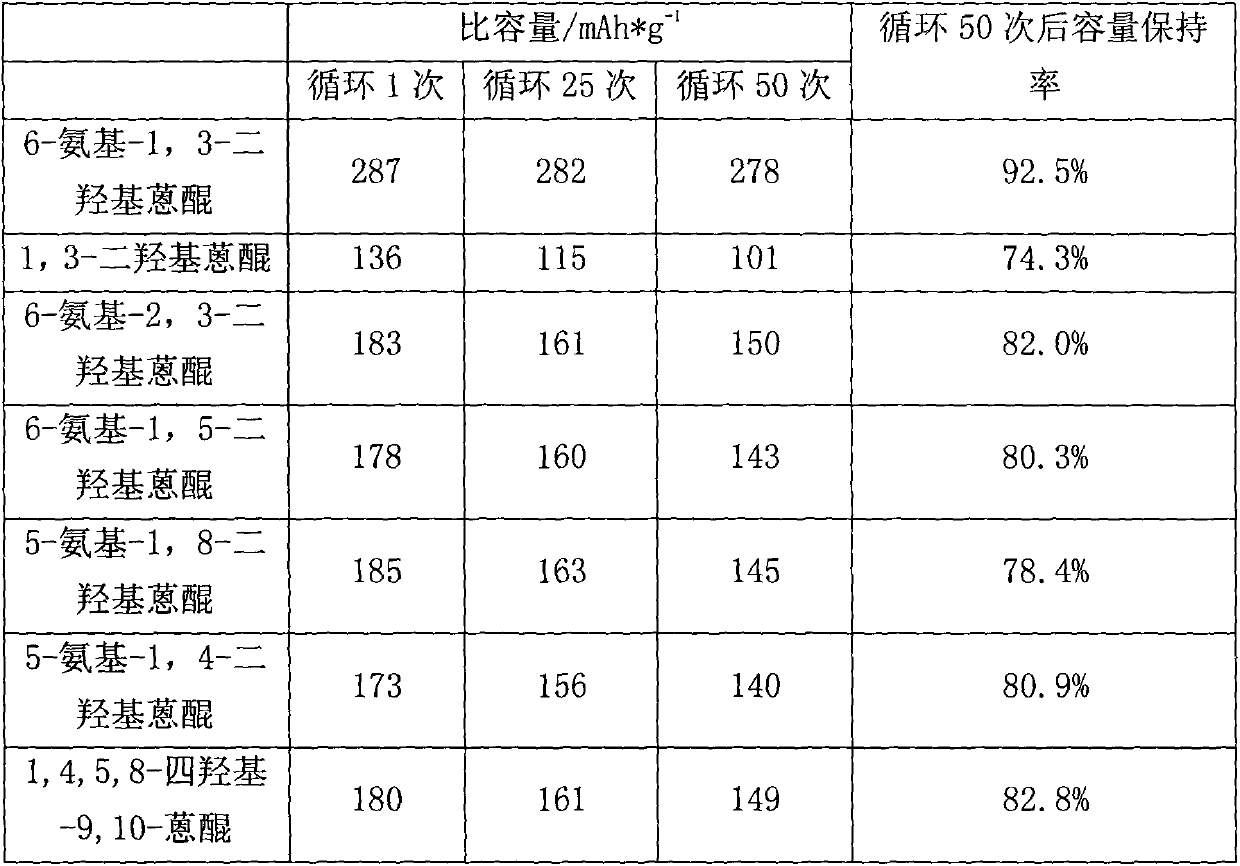
Anthraquinone lithium battery positive pole and preparation method thereof
The invention discloses an anthraquinone lithium battery positive pole and a preparation method thereof. The concrete preparation method comprises the following steps: (1) acetylene black powder is added to a grinding miller and ground for 0.5 hour to 2 hours, and the ground acetylene black powder is sieved by a 1000-mesh sieve; (2) anthraquinone derivatives, the sieved acetylene black powder, N, N-dimethyl formamide (DMF) are mixed, and blended by a mould wash mixer at a room temperature for one hour at the rotation speed of 600 r / min, so that treated carbon dust is obtained; (3) the treated carbon dust is mixed with polyvinylidene fluoride, so that positive pole slurry is obtained; and (4) the slurry is coated on an aluminum foil with a scraper and stoved dry, so that a positive pole sheet is obtained. The anthraquinone lithium battery positive pole is simple in preparation procedure and free from the phase of synthesis of polymers. A lithium battery prepared by means of the positive pole has the advantage of being large in specific capacity.
Owner:北京青晓宏拓医药科技发展有限公司
Aromatic ketones and uses thereof
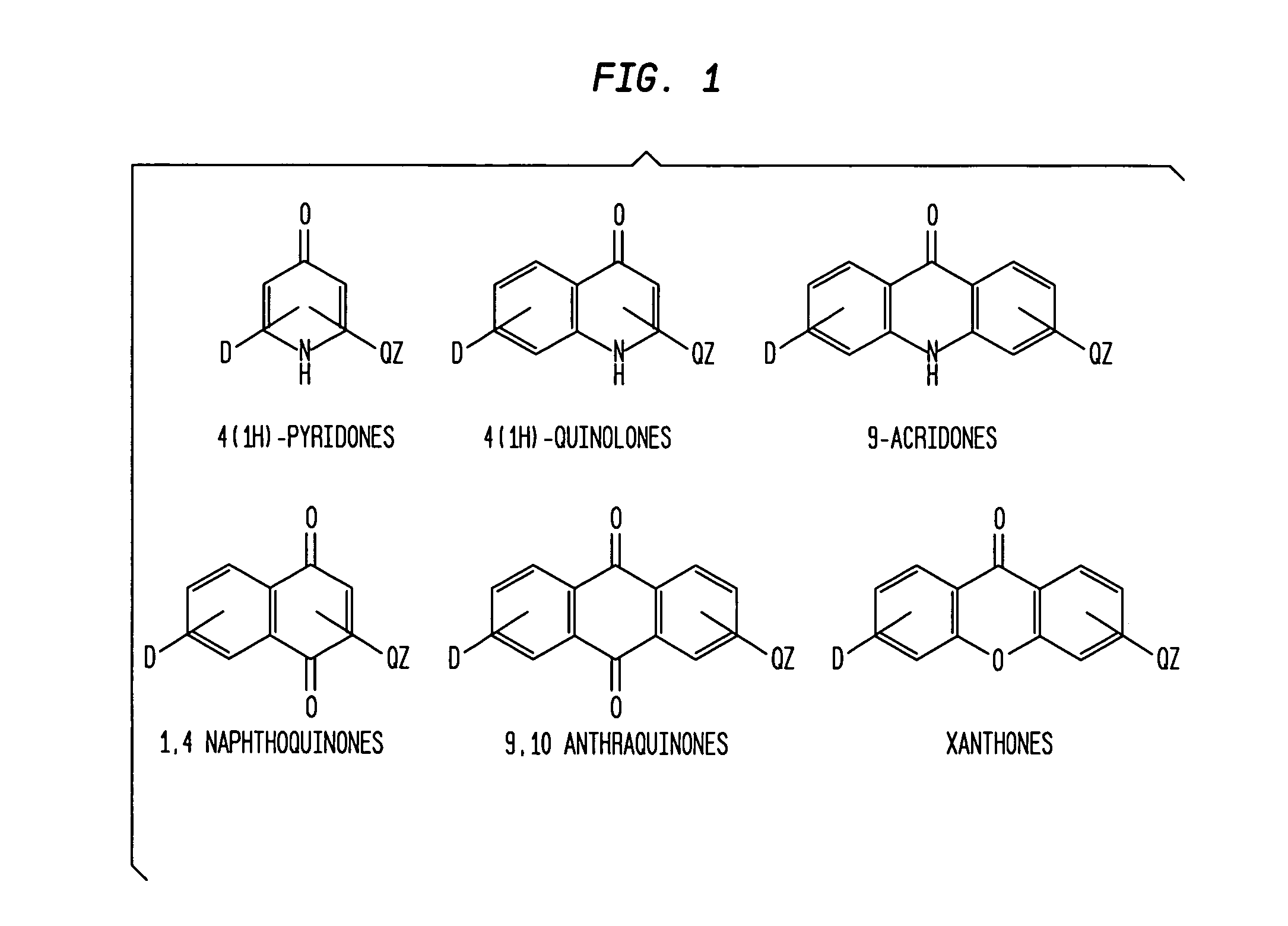
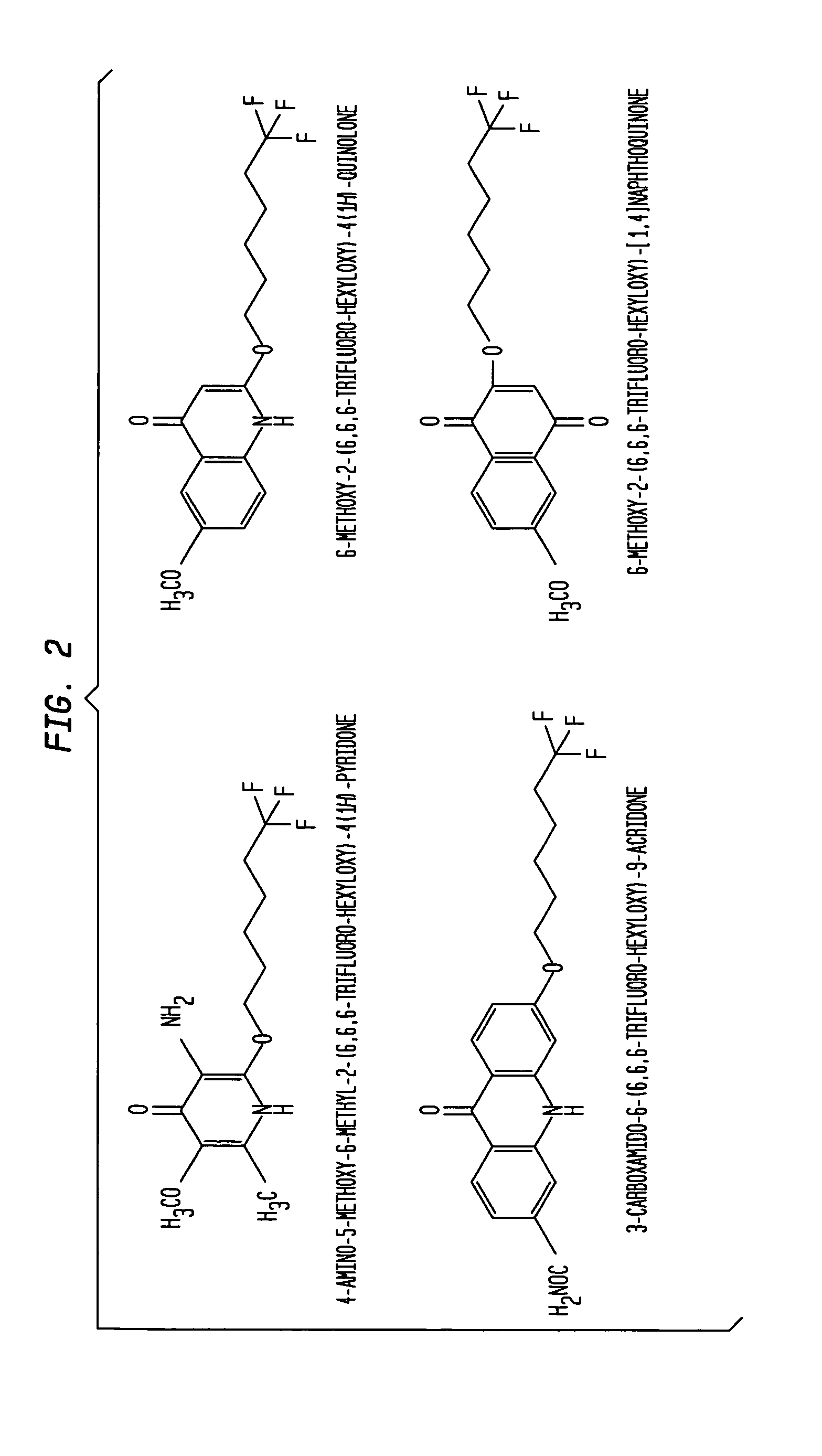
InactiveUS7829578B1Improves antimalarial potencyEnhanced anti-parasiticBiocideOrganic chemistryDiseaseXanthone
Aromatic ketones having an extended fluoro-alkyl or fluoro-alkoxy moiety are disclosed. In particular aspects, the compounds comprise substituted 9-acridone, 9-xanthone, 4(1H)-quinolone, 4(1H) pyridone, 1,4-naphthoquinone, 9,10-anthraquinone derivatives. These preparations possess potent pharmacological activity for inhibiting malaria and mosquito-borne (Plasmodium) diseases. The haloalkyl / alkoxy aromatic compounds possess significant pharmacological activity, with IC50 values in the nanomolar and sub-nanomolar range, and reduced toxicity against host derived cells and tissues. Methods of using the fluoro-alkyl / alkoxy aromatic compounds in the treatment of malaria and other human and animal diseases are also disclosed. Agricultural uses of the fluoro-alkyl / alkoxy aromatic compounds, such as in control of fungal diseases and in the production of important commercial crops (apples, etc.), are also presented.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
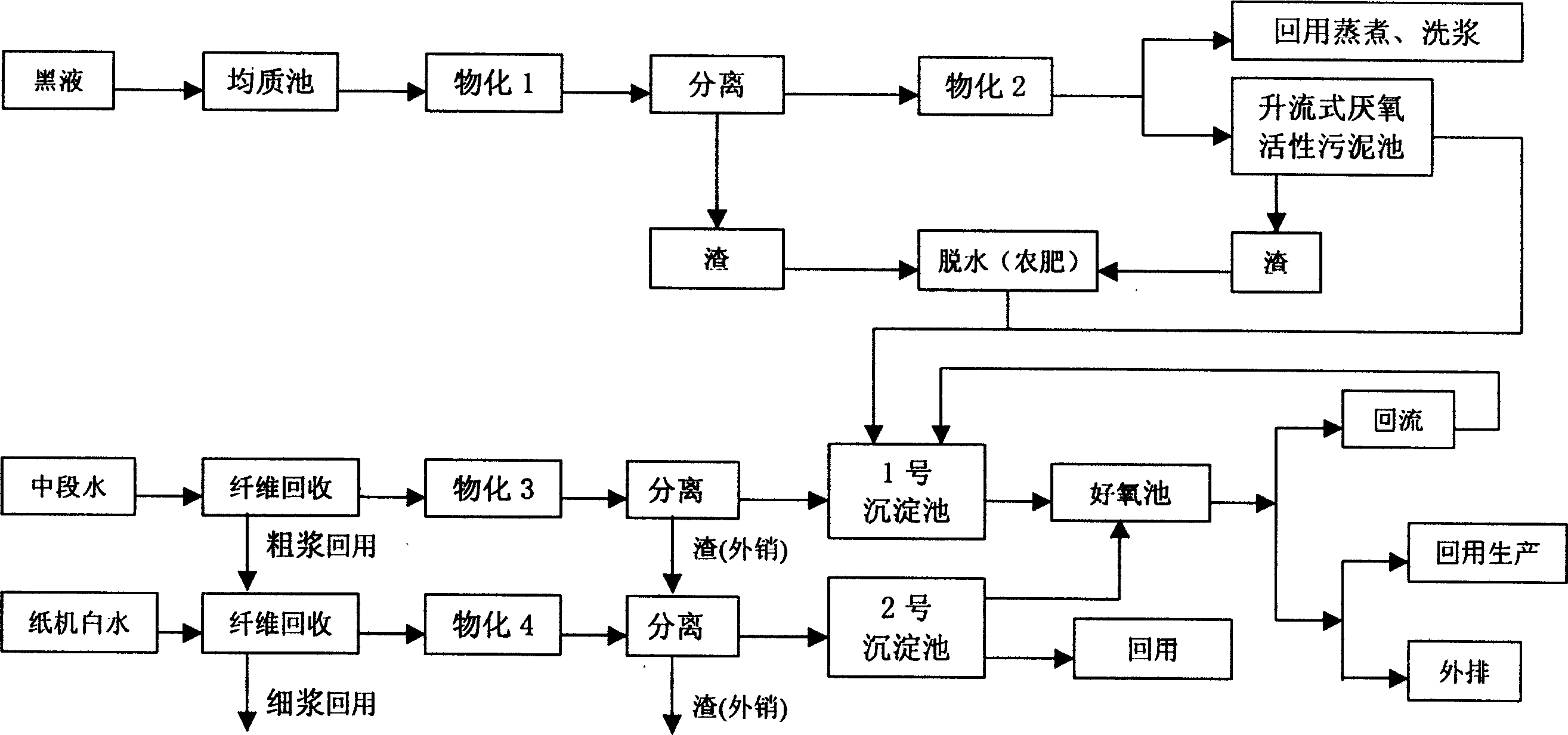
Process for comprensive treating waste water of paper making by grass pulp
InactiveCN1623940AReduce pollutionReduce fine fibersTreatment using aerobic processesTreatment with anaerobic digestion processesEpoxyBlack liquor
A process for treating the sewage generated by making paper with straw pulp includes such steps as preimmersing by alkali method, adding activating agent prepared from anthraquinone derivative, sodium alkylphenylsulfonate and epoxy ethane, fast low-temp digestion, adding 5 kinds of flocculant sequentially for separation, flocculating, decoloring, decomposing lignin and depositing, anaerobic digestion, aerobic aerating, cleaning by biomembrane, and dewatering dregs to obtain fertilizer.
Owner:彭仲辉 +1
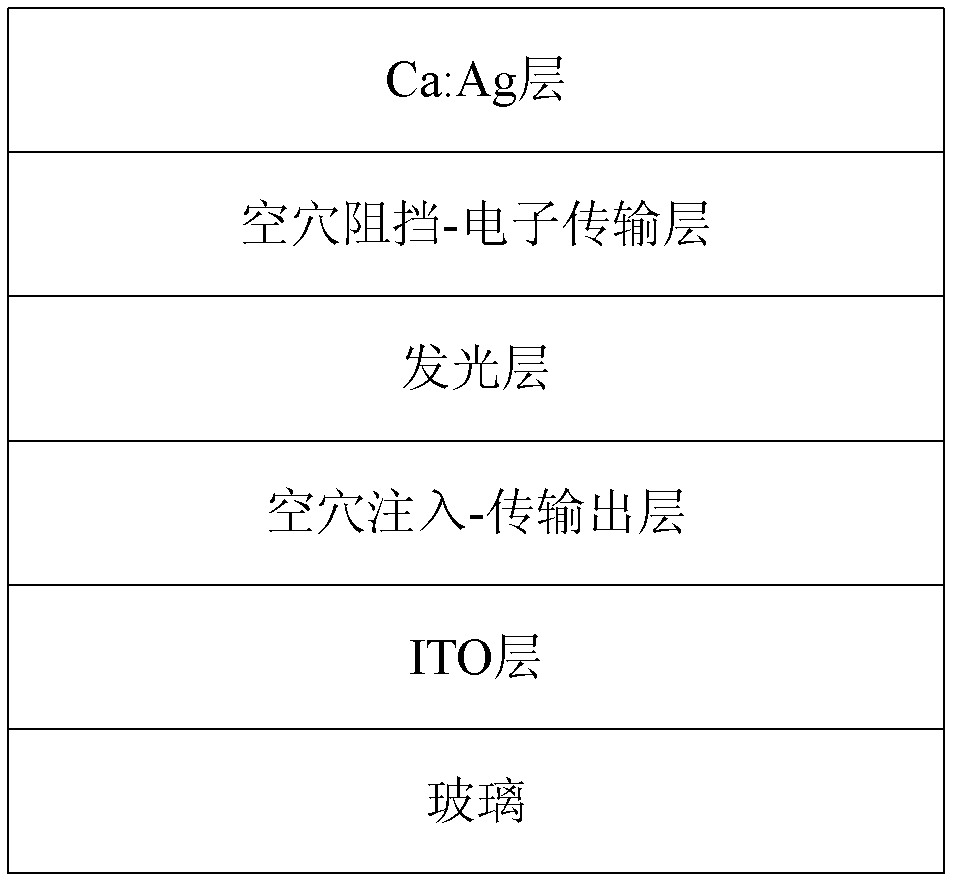
Cyano anthraquinone derivatives, preparation method and organic electroluminescent devices thereof
ActiveCN102786438ASimple preparation processImprove stabilityCarboxylic acid nitrile preparationOrganic compound preparationElectricityHost material
The present invention belongs to the technical field of organic photoelectric materials, and discloses a class of cyano anthraquinone derivatives, a preparation method and an application thereof. The cyano anthraquinone derivatives have the following general formula (I), wherein Ar is an aromatic group. The cyano anthraquinone derivatives of the present invention have characteristics of simple preparation process, good morphology stability, good film forming property, and high singlet state energy level, and are suitable host materials. In addition, electroluminescent devices prepared by adopting the cyano anthraquinone derivatives as a light emitting layer have high luminous efficiency.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
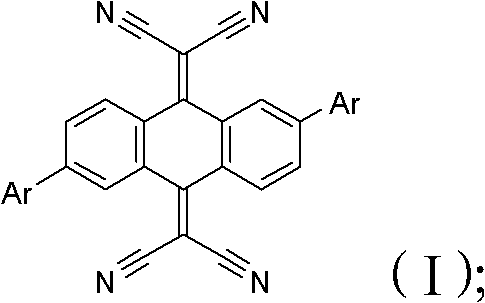
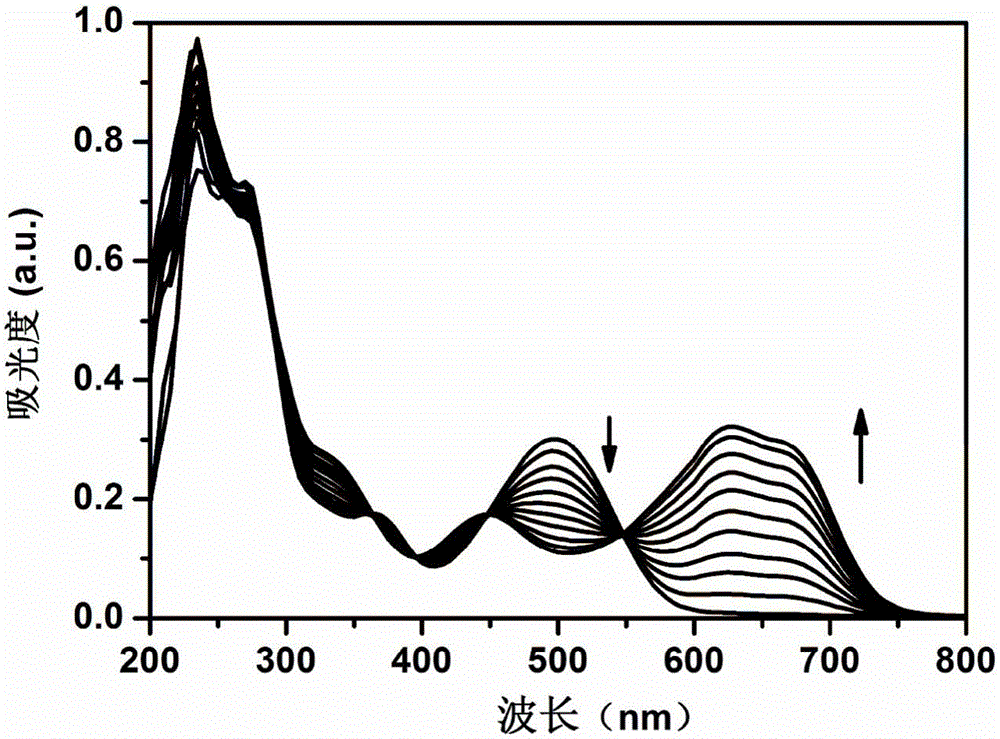
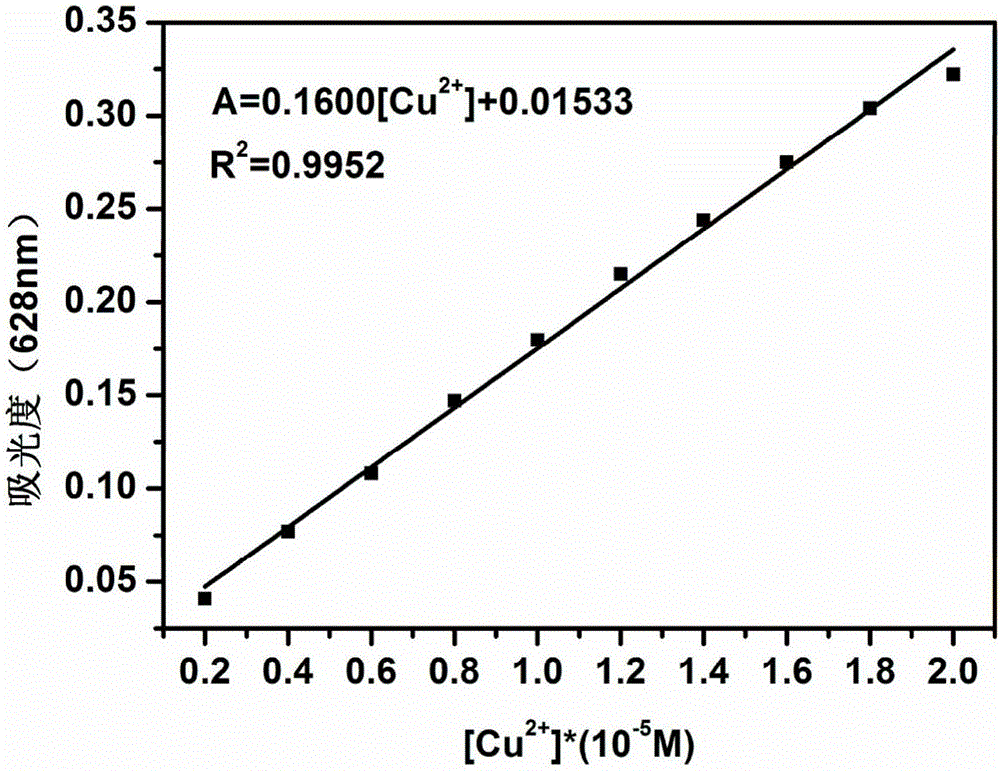
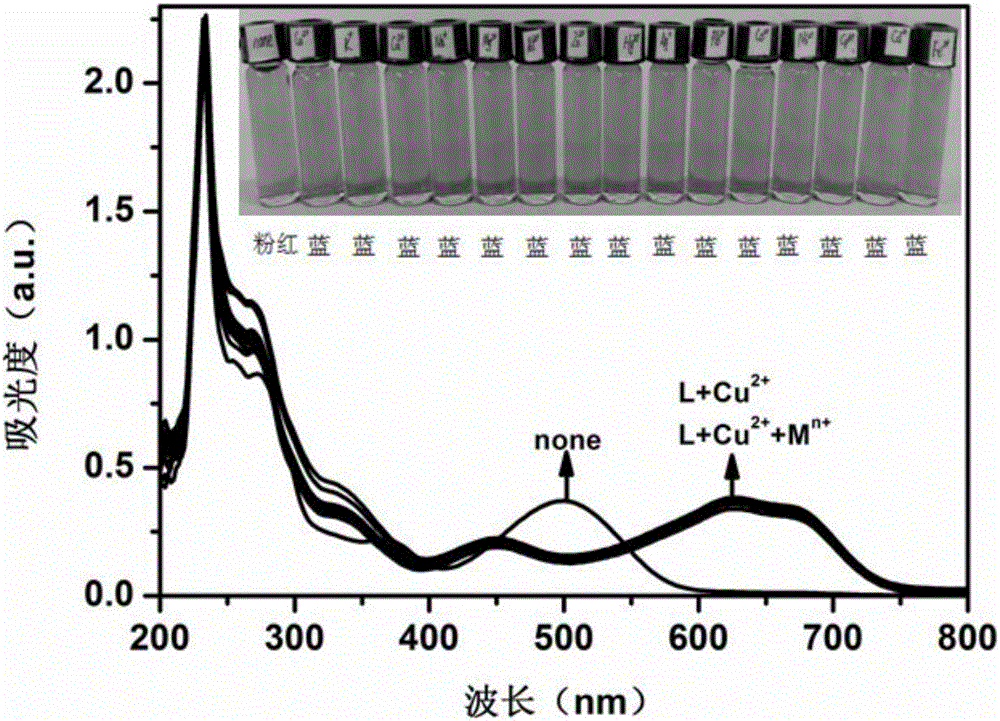
Anthraquinone derivative and synthetic method thereof and application of anthraquinone derivative in detection of Cu<2+>
InactiveCN106045878ALow priceHigh yieldMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSalicylaldehydeFiltration
The invention provides an anthraquinone derivative and a synthetic method thereof and an application of the anthraquinone derivative in detection of Cu<2+>, and belongs to the technical field of anthraquinone derivatives and detection of copper ions. The synthetic method of the anthraquinone derivative comprises: performing heating and refluxing on salicylaldehyde and 1,2-diamino-anthraquinone in methanol, performing suction filtration, and performing washing to obtain the anthraquinone derivative. The invention also provides a method for detecting bivalent copper ions, comprising: quantitatively detecting the content of Cu<2+> in a solution of THF-H2O (pH=7.4) by taking the anthraquinone derivative as a probe. According to the detection method, the copper ions in an aqueous solution can be detected with high selectivity and high sensitivity; and the detection method is simple, convenient and quick in operation. Meanwhile, the invention provides test paper for detecting the bivalent copper ions, so that the Cu<2+> can be detected more conveniently and quickly.
Owner:SHANXI UNIV
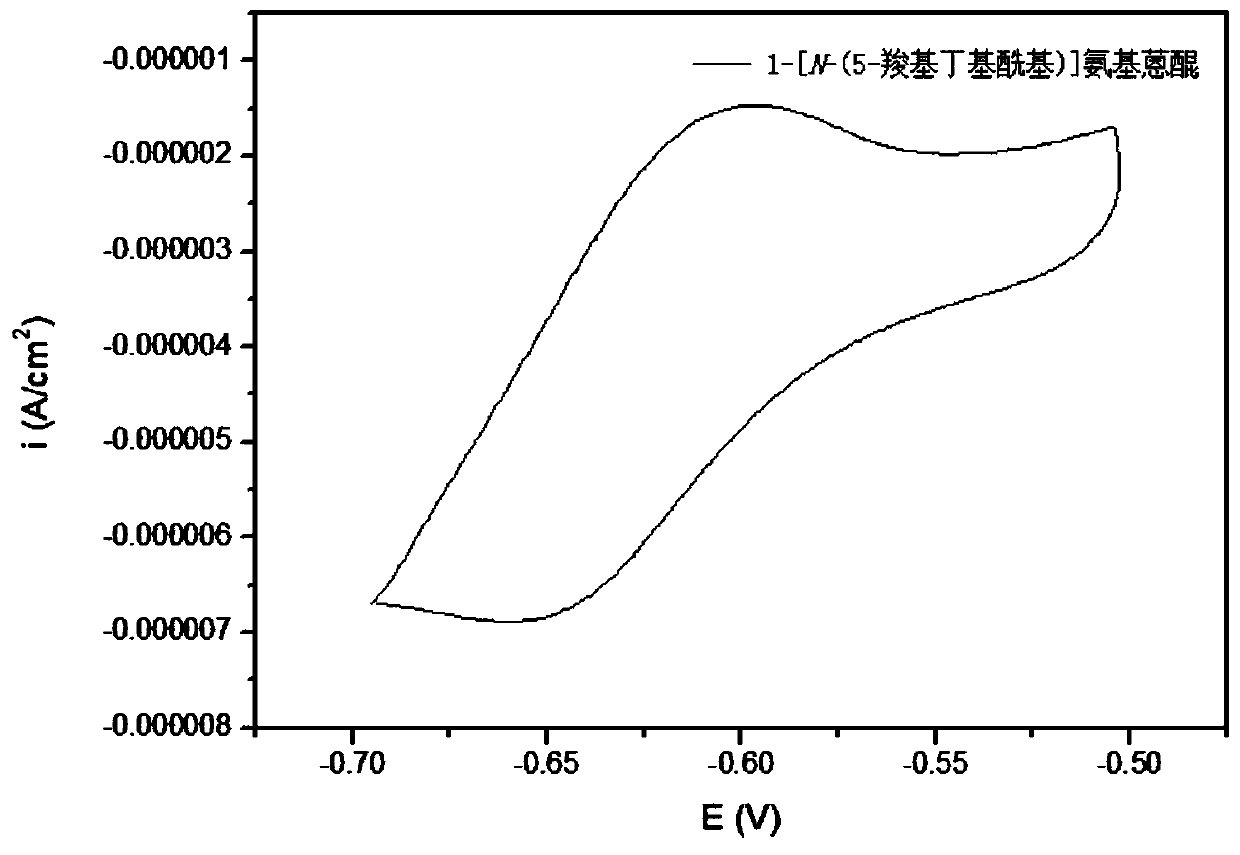
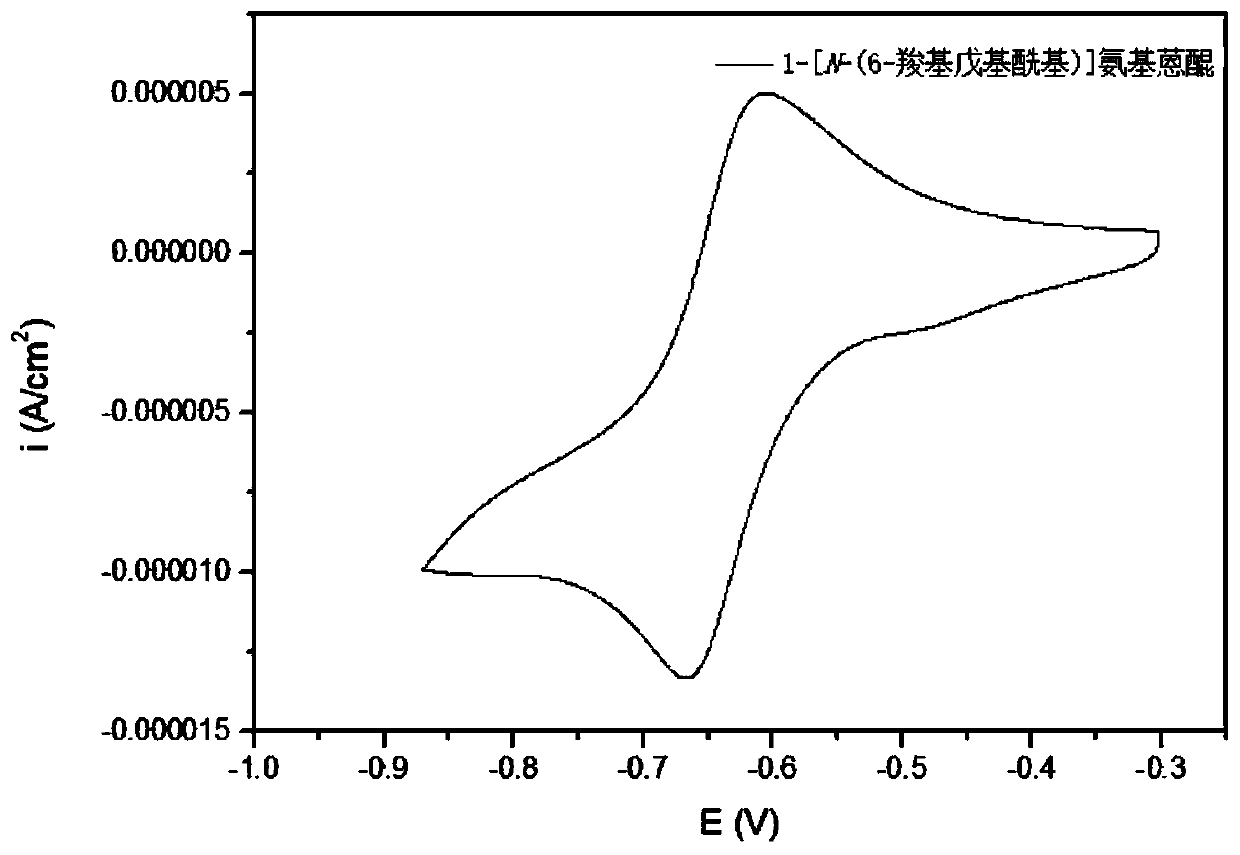
Synthesis method, derivative and battery system of anthraquinone derivative containing carboxyl group
ActiveCN110526826ALow costImprove securityOrganic compound preparationRegenerative fuel cellsCarboxyl radicalDistillation
The invention provides a synthesis method, a derivative and a battery system of an anthraquinone derivative containing a carboxyl group. The synthesis method of the anthraquinone derivative containingthe carboxyl group includes the following steps that S1, dibasic acid containing a terminal carboxyl group and sulfoxide chloride are mixed, toluene is added as a reaction solvent, a catalyst is added, and heated to a set temperature reaction; S2, after the reaction is completed, the solvent and the sulfoxide chloride are removed, the toluene is added for distillation, and a reactant is obtained;S3, the reactant is mixed with amino-anthraquinone, the toluene is added as the reaction solvent, and heating is conducted to a reflux reaction; and S4, after the reaction is completed, the solvent is removed, solids are filtered and removed, the pH value of a filtrate is adjusted to a predetermined value, the solids are separated out, filtered, washed and dried, and the anthraquinone derivativecontaining the carboxyl group is obtained. According to the synthesis method of the anthraquinone derivative containing the carboxyl group, simpleness is achieved, operation is easy, the cost is low,and the synthesis method can be applied to the battery system to solve the problem of electrochemical energy storage.
Owner:CHINASALT JINTAN
Process for the production of hydrogen peroxide
ActiveUS7195748B2Peroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesChemical recyclingAnthranilHydrogen
The invention relates to a process for the production of hydrogen peroxide by the anthraquinone process, comprising a hydrogenation stage, an oxidation stage and an extraction stage. According to the invention, catalytic hydrogenation of anthraquinone derivatives dissolved in a working solution is carried out in the presence of added molecular oxygen. Per mol hydrogen, 0.1 to 10 mmol oxygen is preferably introduced into the hydrogenation stage with the hydrogenating gas, in mixture with an inert gas and / or dissolved and / or dispersed in the working solution. This increases the residence time of the catalyst.
Owner:EVONIK OPERATIONS GMBH
Colored polymeric resin composition, article made therefrom, and method for making the same
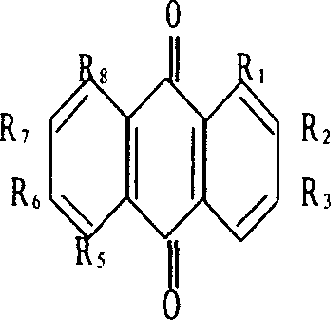
In one embodiment, a colored polymeric resin composition, comprises: a polymeric resin; and a 1,8-anthraquinone derivative having a purity of greater than or equal to about 90 wt % and having a Formula (VIII): wherein R2-R7 are, individually, selected from the group consisting of a hydrogen atom, a hydroxyl group, an alkoxy group, an aryloxy group, an aliphatic group, an aromatic group, a heterocyclic group, a halogen atom, a cyano group, a nitro group, —COR9, —COOR9, —NR9R10, —NR10COR11, —NR10SO2R11, —CONR9R10, —CONHSO2R11, and —SO2NHCOR11; in which R9 and R10 are, individually, selected from the group consisting of a hydrogen atom, an aliphatic group, an aromatic group, and a heterocyclic group; wherein R11 is selected from the group consisting of an aliphatic group, an aromatic group, and a heterocyclic group; and wherein R is selected from the group consisting of hydrogen, an alkyl group containing 1 to 20 carbon atoms, a cycloalkyl group containing 3 to 20 carbon atoms, an allyl group containing 3 to 20 carbon atoms, a hydroxyl group, a 5-membered heterocyclic ring, and a 6-membered heterocyclic ring.
Owner:SABIC GLOBAL TECH BV
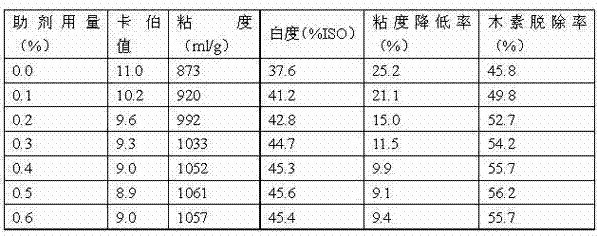
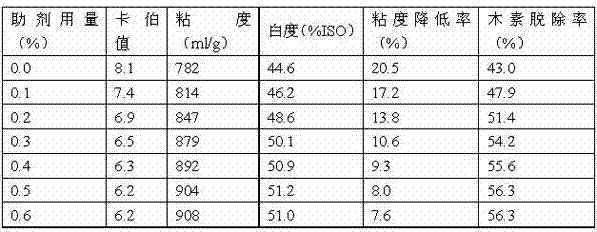
Accessory ingredient used for bleaching slurry oxygen delignification and application thereof
ActiveCN102817266AReduce surface tensionImprove permeabilityCellulosic pulp after-treatmentFiberSulfite salt
The invention discloses an accessory ingredient used for bleaching slurry oxygen delignification, which aims to solve the problems that the traditional oxygen delignification technology adopts magnesium sulfate or sodium tripolyphosphate as fiber protection accessory ingredient to cause single chemical component and poor action effect, and can not perform good delignification action, and the improvement of the whiteness of the subsequent bleaching slurry is affected due to uneven reaction of oxygen and lignin. The accessory ingredient comprises the following components in percentage by weight: 5-15% of sodium dodecyl benzene sulfonate, 3-20% of sodium lignosulphonate, 5-25% of anthraquinone derivative, 25-65% of sodium sulfite and 1-15% of sodium tripolyphosphate. The accessory ingredient disclosed by the invention is compounded by various components, the delignification rate, the slurry yield and the slurry whiteness can be effectively improved, and the fiber is prevented from being degraded. Meanwhile, the use amount of bleaching agent in the subsequent bleaching process is effectively reduced, pollution on the environment by bleaching waste water is lowered, and the accessory ingredient has a higher economic value and a higher use value.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Anthroquinone containing derivatives as biochemical agricultural products
Formulations containing anthraquinone derivatives with increased effectiveness as pesticides are provided. These formulations may comprise (a) a preparation comprising one or more anthraquinone derivatives having activity against plant pests; (b) one or more C2-C7 alcohols, or glycols or lactones; and (c) one or more surfactants selected from the group consisting of a sulfate, ethoxylated fatty acid esters wherein said alcohols and surfactants are present in amounts effective to stability said preparation. Also provided are methods of using these formulations as pesticides.
Owner:POINT FINANCIAL
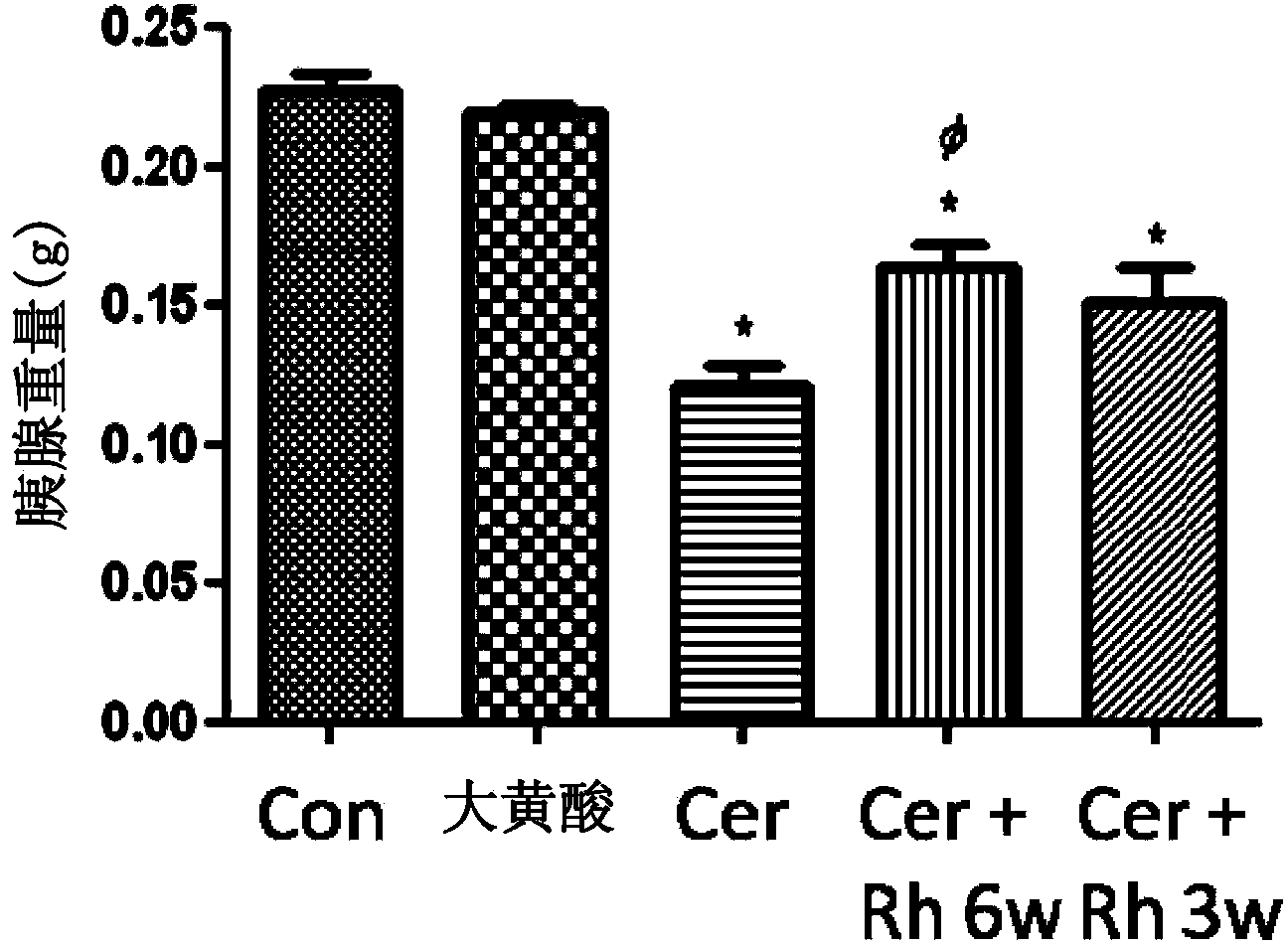
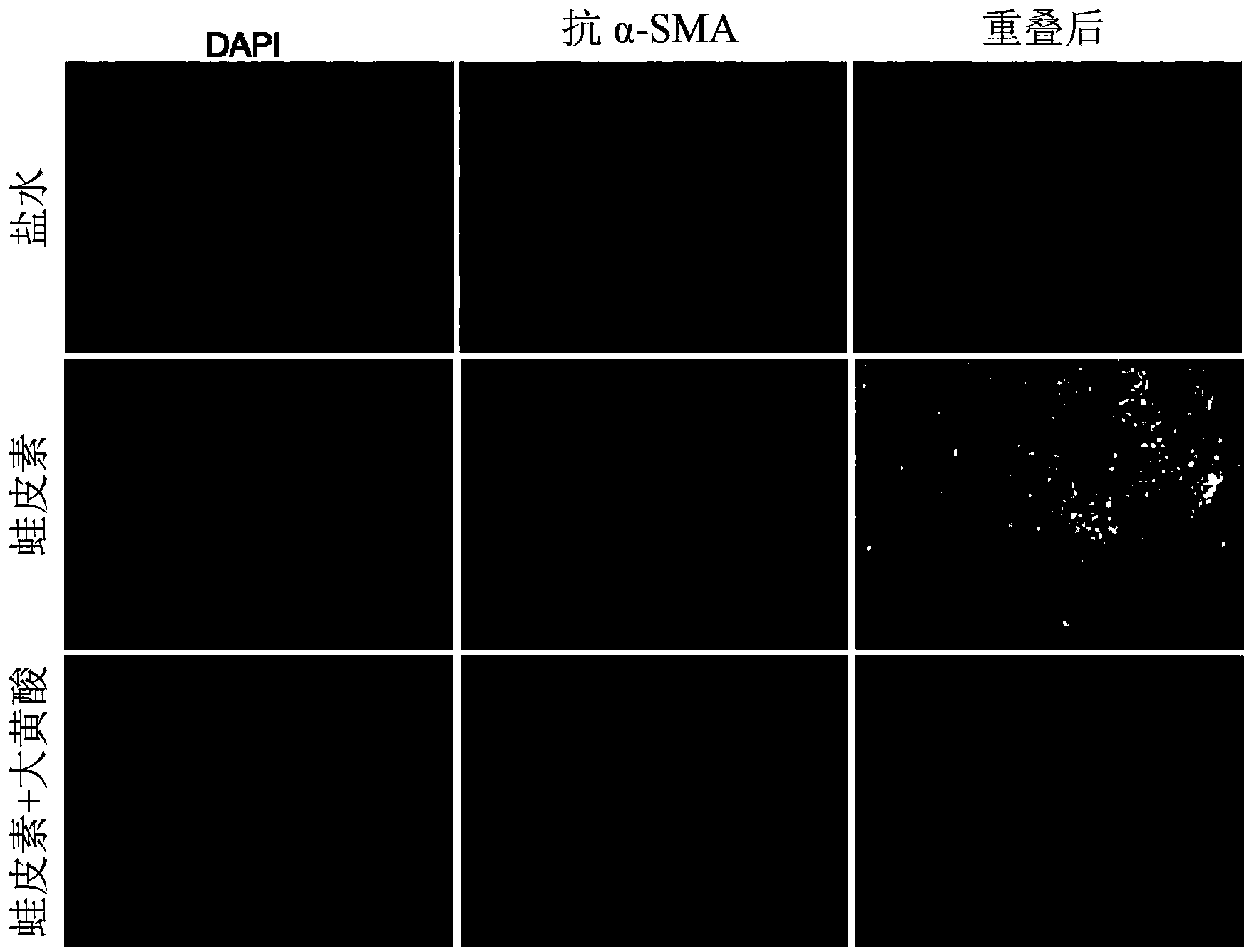
Method of using rhein for treating fibrotic conditions and tumors
The present invention relates to a method of using an anthraquinone derivative namely 9,10-Dihyro-4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylic acid, or known as Rhein, for treating chronic pancreatitis induced fibrosis of the pancreas. More particularly, the present invention relates to a method of using Rhein, its derivatives and / or chemical variants as an anti-fibrotic agent. The present invention particularly relates to the suppression of pancreatic stellate cell activation for the management of chronic inflammatory, fibrotic and tumorigenic pathologies in the pancreas.
Owner:HONG KONG BAPTIST UNIV
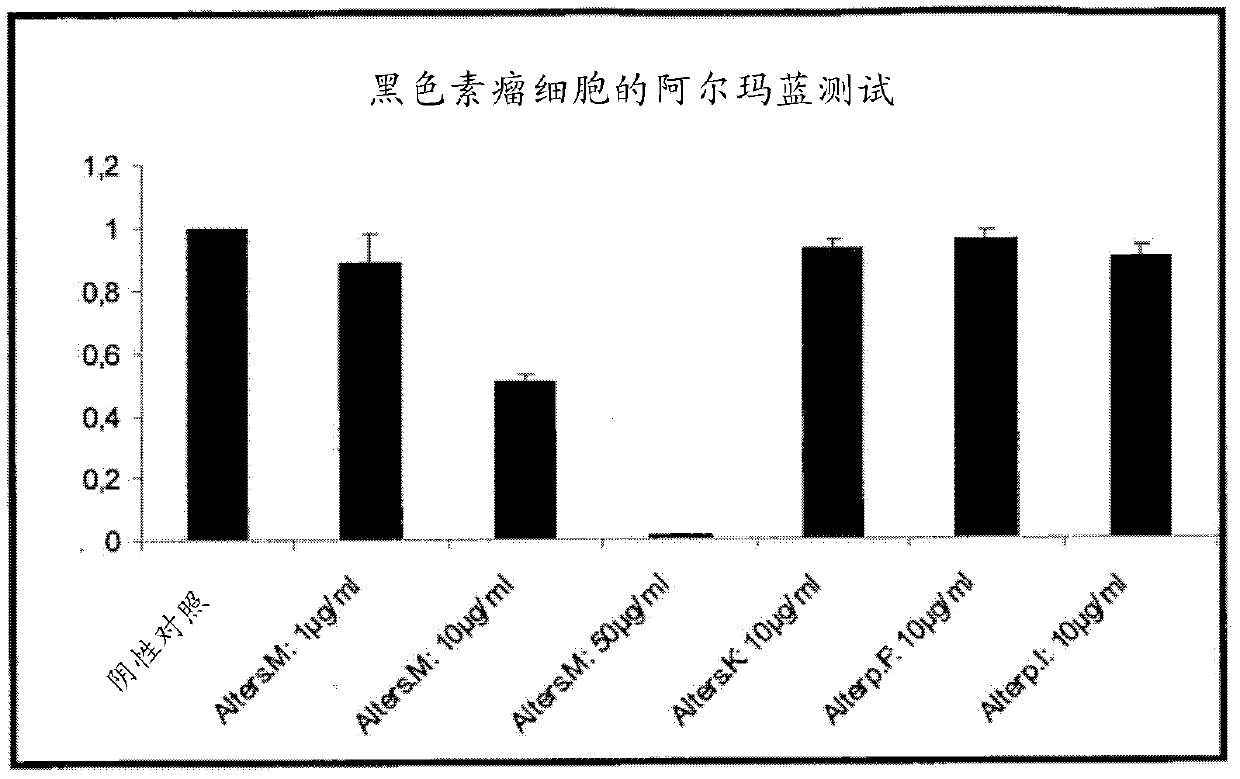
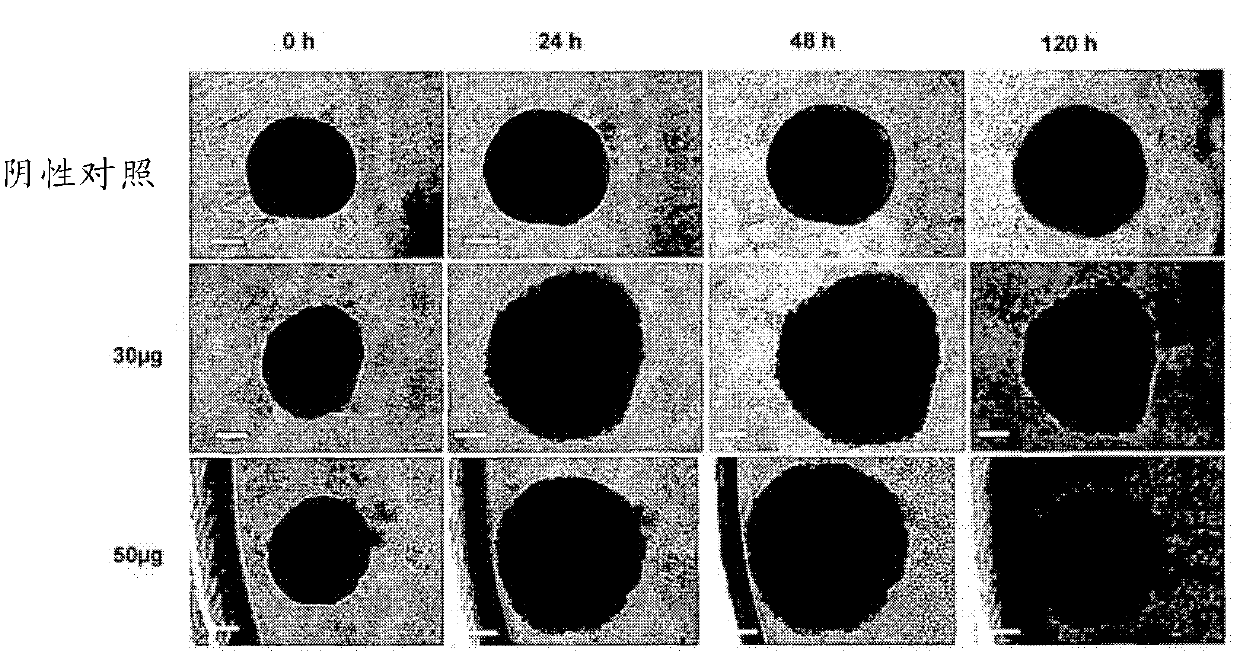
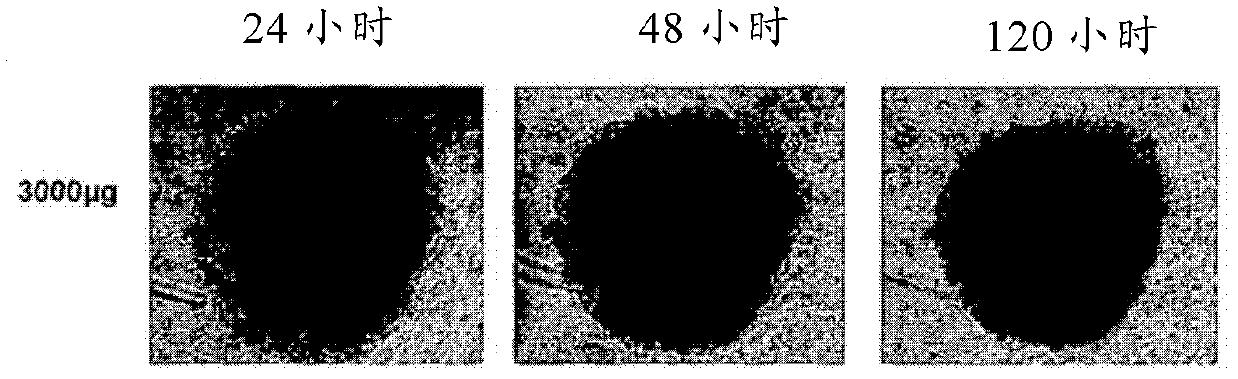
Novel anthraquinone derivatives
InactiveCN102482188AIncrease productionHigh purityAntibacterial agentsOrganic active ingredientsAnthraceneAnthranil
The invention relates to two novel substances, specifically (1R,2S,3S,4R)-3-acetoxy-1,2,4,5-tetrahydroxy-y-methoxy-2-methyl-1,2,3,4-tetrahydroanthracene-9,10-dione (3-O-acetyl-altersolanol M) (I) and 8-(4,5,6-trihydroxy-7-methyl-2-methoxy-9,10-dioxo-9H,10H-anthracene-1-yl)-(1S,2S,3R,4S)-1,2,3,4,5-pentahydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydro-9H,10H-anthracene-9,10-dione (atropisomeric alterporriol I und J), (II) to the use thereof as anti-infective agents or anti-cancer agents, and to a production method therefor.
Owner:SEALIFE PHARMA
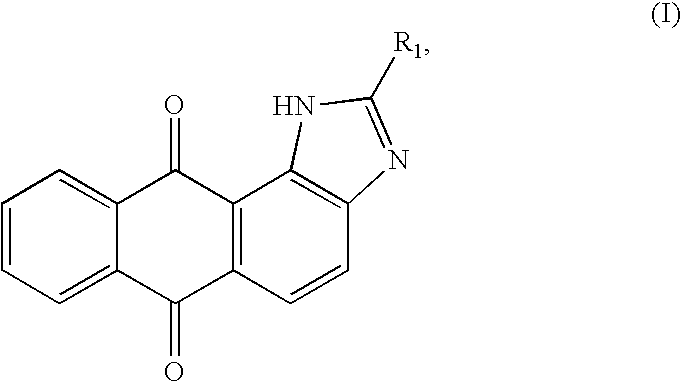
Heteroannelated anthraquinone derivatives and the synthesis method thereof
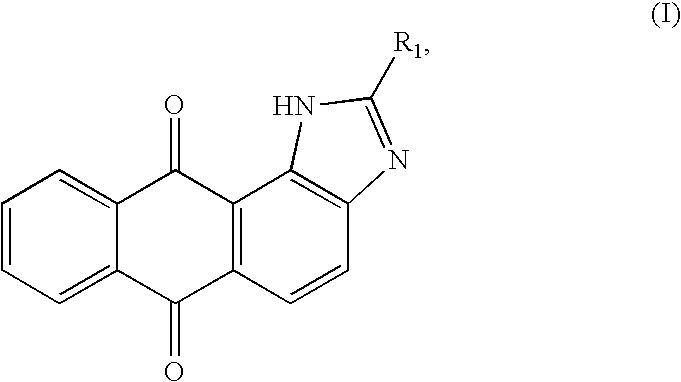
InactiveUS20090253707A1Easy to getIncrease ratingsBiocideOrganic chemistrySynthesis methodsDouble bond
A heteroannelated anthraquinone derivative compound is provided. The heteroannelated anthraquinone derivative compound is represented by a formula (I):wherein R1 is a substituent being one selected from a group consisting of i) a first substituent being one selected from a group consisting of a hydryl group, an amino group, a nitro group, a hydroxyl group and a cyan group, ii) a second substituent being one selected from a group consisting of (CH2)nX, a straight (CH2)n alkyl group, a (CH2)n alkoxyl group, a branched (CH2)n alkyl group, a C3˜C12nephthenic group, and a C3˜C12 cyclic alkoxyl group, wherein 1=n=12, and X is a halogen, iii) a third substituent being one selected from a group consisting of a straight C1˜C8 alkyl group with a double-bond, a C1˜C8 alkoxyl group with a double-bond, a branched C1˜C8 alkyl group with a double-bond and a C3˜C8 nephthenic group with a double-bond, and iv) a fourth substituent of a C5˜C12 heterocyclic group.
Owner:NAT DEFENSE MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com