Application of compound to preparation of medicines for treating viral pneumonia
A technology for viral pneumonia and compounds, applied in the field of medicine, can solve problems such as lack of new antiviral drugs, and achieve the effects of superior antiviral pneumonia effect, simple preparation method and significant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
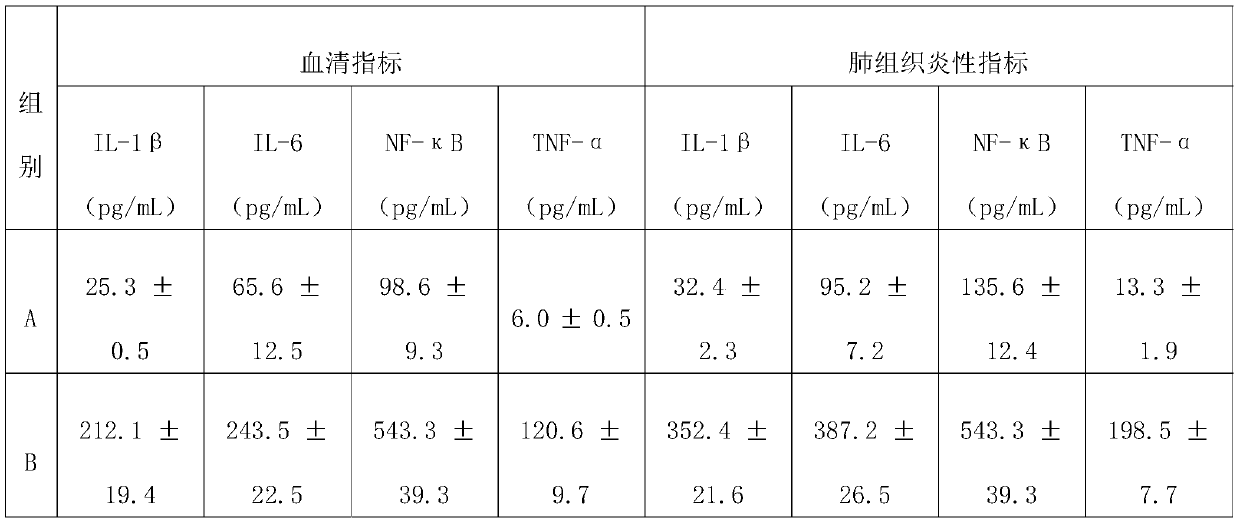
[0024] This example provides the effect of combined drugs on coronavirus pneumonia.
[0025] Experimental method: C57BL / 6 mice (20-25g) were randomly divided into 5 groups according to different body weights, namely: group A, blank control group; group B, HcoV-OC43 virus model group; group C, HcoV-OC43 virus model group Compound 1 group; D group, HcoV-OC43 virus compound 2 group; E group, HcoV-OC43 virus compound 1 and 2 combination group (low dose); F group, HcoV-OC43 virus compound 1 and 2 combination group (high dose) ; Group G, positive drug ribavirin group; H group, positive drug oseltamivir group. Animals in all groups were anesthetized with propofol tail vein, except for the blank control group, they were all infected with HcoV-OC43 virus solution (30 μL) by intranasal drip.
[0026] Subsequent processing is as follows:
[0027] Group A mice: intragastric administration of the same dose of normal saline in the drug intervention group;
[0028] Group B mice: intragast...
Embodiment 2
[0042] This example provides the effect of combined drugs on influenza A virus pneumonia.
[0043] Experimental method: C57BL / 6 mice (20-25g) were randomly divided into 5 groups according to body weight: Group A, blank control group, group B, HcoV-OC43 virus model group, group C, HcoV-OC43 virus compound Group 1, group D, HcoV-OC43 virus compound group 2, group E, HcoV-OC43 virus compound 1 and 2 combination group (low dose), F group, HcoV-OC43 virus compound 1 and 2 combination group (high dose), Group G, positive drug ribavirin group, group H, positive drug oseltamivir group. Animals were anesthetized with propofol through the tail vein, and were infected with H1N1 virus solution (30 μL) by intranasal drip except for the blank control group. Subsequent operations:
[0044] Group A mice: The intervention group was given the same dose of normal saline by intragastric administration of drugs;
[0045] Group B mice: intragastric administration of drugs to give the interventio...
Embodiment 3
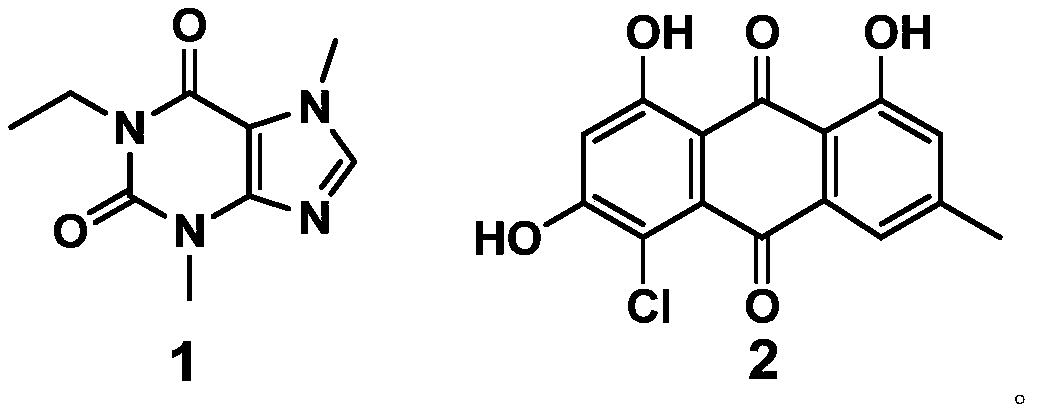
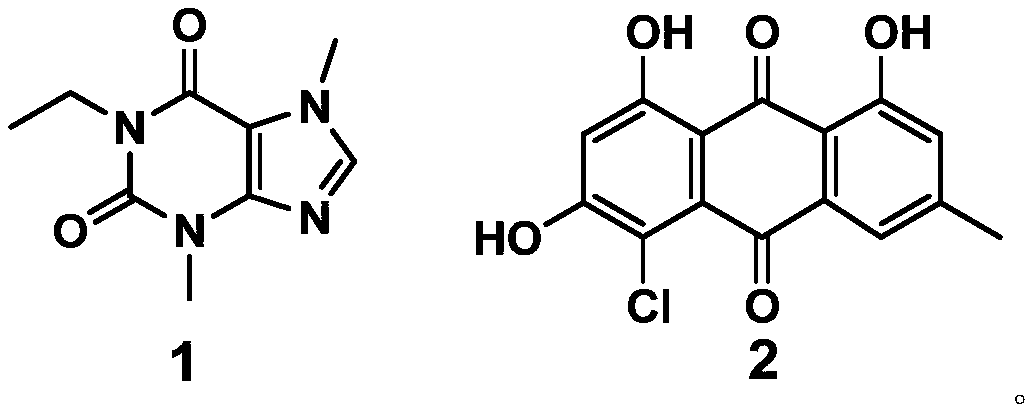
[0059] This example provides a synthesis method of the purine compound (1-ethyl-3-7-dimethylxanthine) with the structure of formula 1.
[0060] Take 540mg (3mmoL) of theobromine and 720mg (30mmol) of NaH dissolved in 80mL of DMF, and add 2mL of iodoethane (24.7mmol) dropwise under stirring. After reacting for 4 hours, add water to quench the reaction, spin dry under reduced pressure, and separate on a silica gel column (mobile phase; petroleum ether: CH 3 CH 2 OCOCH 3 =3:1) The product compound 1-ethyl-3-7-dimethylxanthine (495mg, 2.5mmol, 85%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com