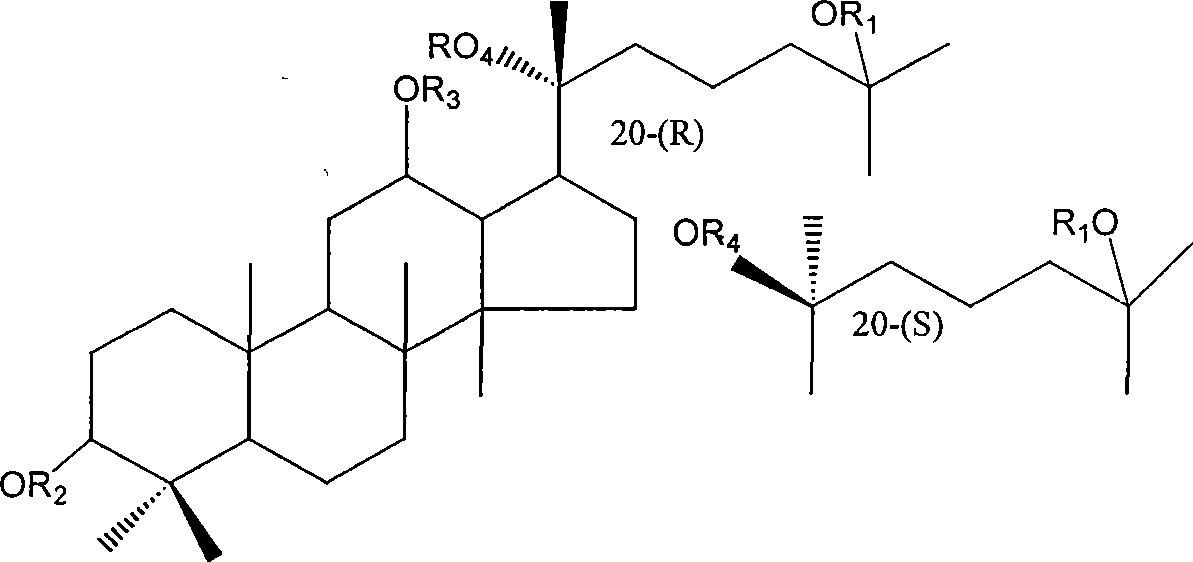
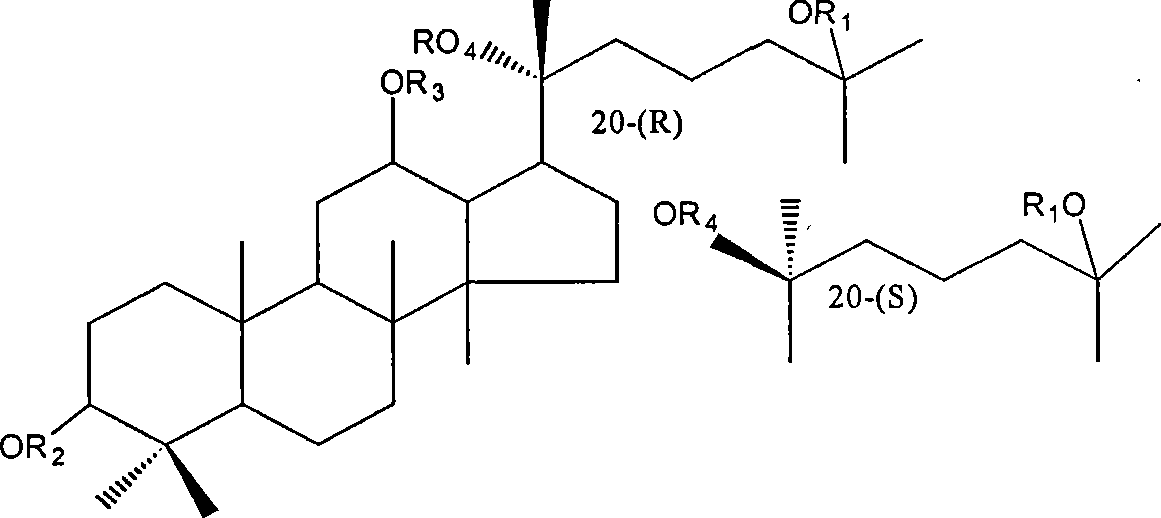
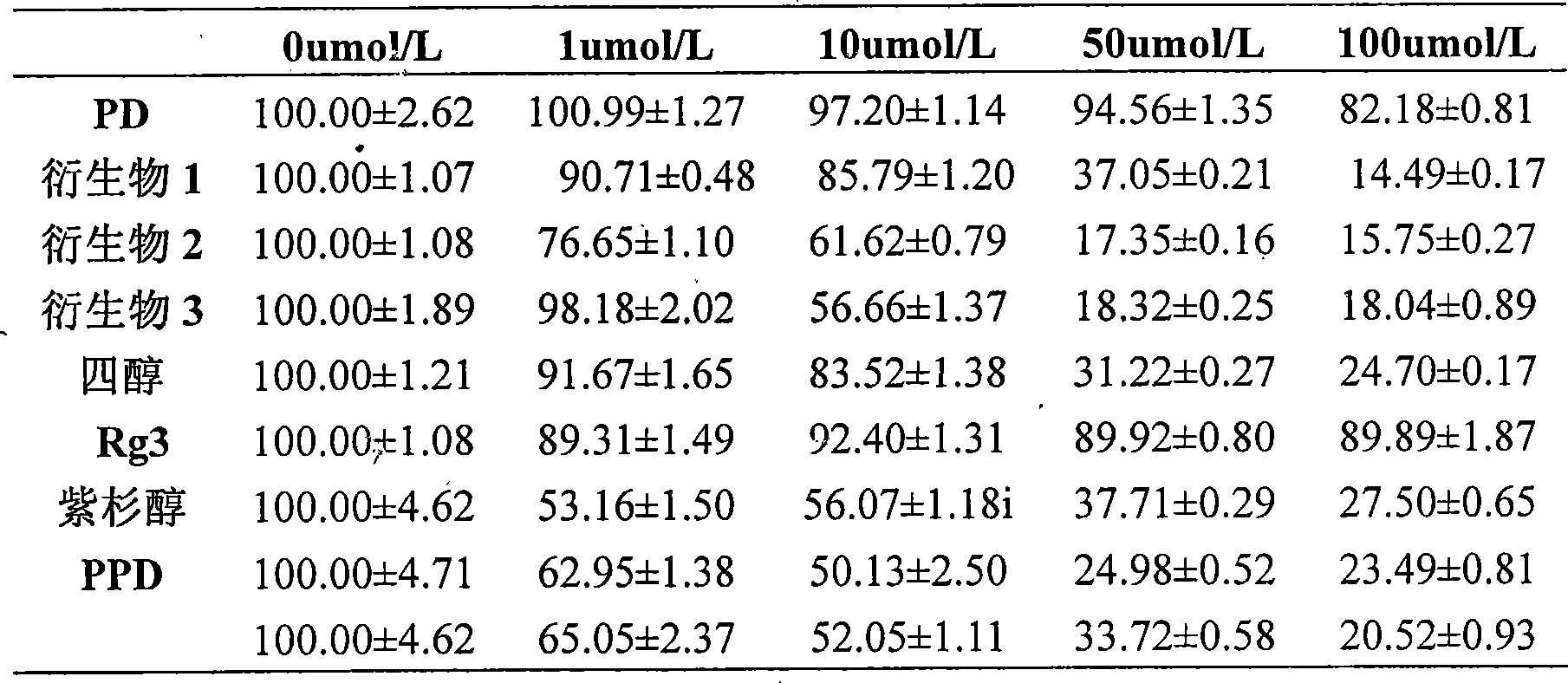
20(S) or 20(R)-dammarane-3 beta,12 beta, 20,25-tetraalcohol derivative, its salt and use thereof
A technology of dammarane and derivatives, which is applied in the field of medicine to achieve the effect of reducing activity and strong anti-virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of dammarane-3β, 12β, 20,25-tetraol-12β-monomethyl ether and dammarane-3β, 12β, 20,25-tetraol-3β, 12β-dimethyl ether
[0033] 0.3g of dammarane-3β,12β,20,25-tetraol was dissolved in 15ml of DMF, and 0.3g of sodium hydride (60%) was added. After all the sodium hydride was dissolved, 0.2ml of methyl iodide was added, heated to 70°C, and refluxed for 8 hours. An appropriate amount of water was added to the reaction solution, and a white solid was precipitated. The precipitated solid was filtered, dried, and subjected to silica gel column chromatography, eluting with petroleum ether: acetone (3:1), to obtain two components: component 1 was a white oily dammarane-3β, 12β, 20, 25-tetra Alcohol-3β, 12β-dimethyl ether (69.7%), Fraction 2 was dammarane-3β, 12β, 20,25-tetraol-12β-monomethyl ether (48.6%) as a white solid. Example 2: Preparation of dammarane-3β, 12β, 20,25-tetraol-12β-monoethyl ether and dammarane-3β, 12β, 20,25-tetraol-3β, 12β-diethyl eth...
Embodiment 2
[0034] 0.5 g of dammarane-3β, 12β, 20, 25-tetraol was dissolved in 15 ml of DMF, and 0.5 g of sodium hydride (60%) was added. After all the sodium hydride was dissolved, 0.4 ml of ethyl bromide was added, heated to 75°C, and refluxed for 10 hours. An appropriate amount of water was added to the reaction solution, and a white solid was precipitated. The solid was separated out by filtration, dried, and subjected to silica gel column chromatography, eluting with petroleum ether: ethyl acetate (4:1), to obtain two components: component 1 was a white oily dammarane-3β, 12β, 20, 25 - Tetrol-3β, 12β-diethyl ether (25.2%), Fraction 2 is a white solid dammarane-3β, 12β, 20,25-tetraol-12β-monoethyl ether (47.3%).
Embodiment 3
[0035] Example 3: Preparation of dammarane-3β, 12β, 20,25-tetraol-12β-n-pentyl ether
[0036] 0.4 g of dammarane-3β, 12β, 20, 25-tetraol was dissolved in 18 ml of DMF, and 0.4 g of sodium hydride (60%) was added. After all the sodium hydride was dissolved, 0.3 ml of n-bromoalkane was added, heated to 80°C, and refluxed for 5 hours. An appropriate amount of water was added to the reaction solution, and an oily substance was precipitated, which was left overnight. The supernatant was decanted, washed once with water, and the oil was extracted with chloroform, and the extract was dried with anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give dammarane-3β,12β,20,25-tetraol-3β,12β-n-pentyl ether (89.5%) as a pale yellow oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com