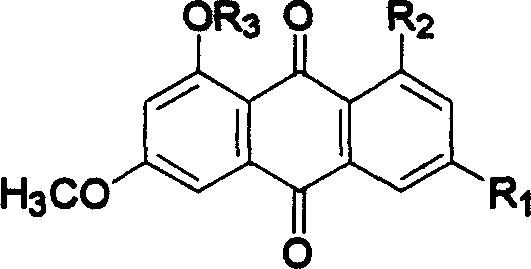
Hydroxyanthraquinone derivatives and their application in preparation of anticancer medicines
A technology of hydroxyanthraquinones and derivatives, applied in the field of hydroxyanthraquinones derivatives, can solve the problem of insufficient anti-tumor activity and achieve strong cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
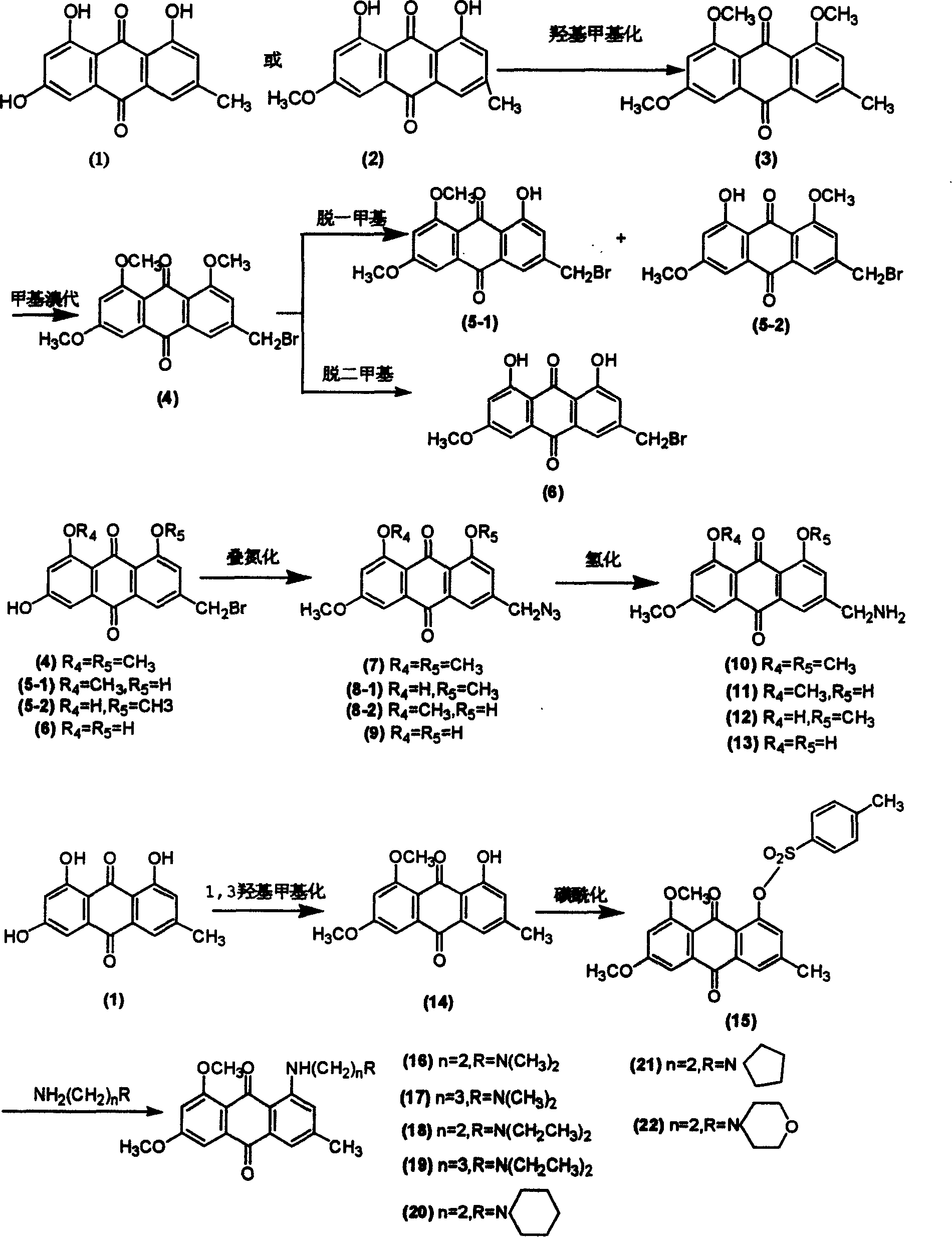
[0021] Example 1: Extraction, separation and purification of natural hydroxyanthraquinone compounds emodin and emodin methyl ether
[0022] Weigh 1kg tiger stick, crush it through a 20-mesh sieve, use 95% industrial alcohol, reflux and extract twice on a water bath, combine the filtrate, concentrate it under reduced pressure to 500ml, add 100-500ml of 3Mol. water, stand overnight, and dry by suction filtration to obtain 180g pink powder. Put the obtained red powder into a Soxhlet extractor and extract with ether until the ether is close to colorless in the Soxhlet extractor. )NaHCO 3 solution (6×100ml), the ether layer was extracted, and the aqueous phase was discarded. 5% (w / w) Na 2 CO 3 ) solution (8 × 100ml), extract the ether layer, 2 × 100ml of the extract before merging was adjusted to PH3 and elemental analysis data.
[0023] Emodin, orange-red needle-like crystals, m.p.254-256℃IR(KBr).3300, 1675, 1625; 1 H NMR (DMSO-d 6 / TMS, 500 MHz) δ: 2.351(s, 3H), 6.503(d, J=...
Embodiment 2
[0026] Embodiment two: the preparation of intermediate product (3)
[0027] Weigh 4.2g (15.6mmol) emodin, anhydrous K after grinding 2 CO 3 16g (28.6mmol), add Me 2 SO 4 5ml (50mmol), 380ml of acetone, stirred and refluxed for 5-20h, concentrated, stirred with water, filtered, washed with a small amount of cold acetone to obtain 4.4g of light yellow powder (3), yield 90.5%. Product structure by IR, MMR, M 3 and elemental analysis data. m.p. 225-227°C, IR(KBr)v: 1661, 1600, 1564, 1321, 1245. 1 H NMR (CDCl 3 / TMS, 500MHz) δ: 2.467(s, 3H), 3.950(s, 3H), 3.962(s, 3H), 3.984(s, 3H), 6.763(d, J=2.5, 1H), 7.094(s, 1H), 7.320(d, J=2.5, 1H), 7.640(s, 1H); FAB-Ms m / z 313[M+1] + ;Anal.Calcd for C 18h 16 o 5 : C 69.22, H 5.16, found C 69.31, H 5.12.
Embodiment 3
[0028] Embodiment three: the preparation of intermediate product (4)
[0029] Weigh 4.0g (6.4mmol) and 2.0g (3.5mmol), 1,3-dibromo-5,5-dimethylcaprolactin (BDH), add 200-500ml CCl 44 , after thermally dissolving, add 0.6g benzoyl peroxide, stir and reflux for 6-15h, filter, wash the solid with hot water twice, dry, and separate by silica gel column chromatography (ethyl acetate:benzene=10:90) to obtain Light yellow powder (4) 2.8g, yield 56.0%. Product structure by IR, MMR, M 3 and elemental analysis data. m.p 250-252℃, IR(KBr)υ: 1665, 1597, 1334, 1245; 1 HNMR (CDCl 3 / TMS, 500MHz) δ: 3.962(s, 3H), 3.971(s, 3H), 4.023(s, 3H), 4.523(s, 2H), 6.779(d, J=2.5, 1H), 7.309(s, 1H), 7.328(d, J=2.5, 1H), 7.832(s, 1H); FAB-Ms m / z 391[M+1] + ;Calcd for C 18 h 15 BrO 5 : C 55.26, H 3.86, found C 55.38, H 3.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com