Application of emodin anthraquinone derivative in preparation of anti-hepatocellular carcinoma drugs
A technology of anthraquinone derivatives and emodin, which is applied in drug combinations, antineoplastic drugs, and pharmaceutical formulations, can solve the problems of difficult extraction, limited structure optimization, activity screening, and poor bioavailability, and achieve obvious cytotoxicity , Inhibition of tumor cell growth, significant anti-liver cancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
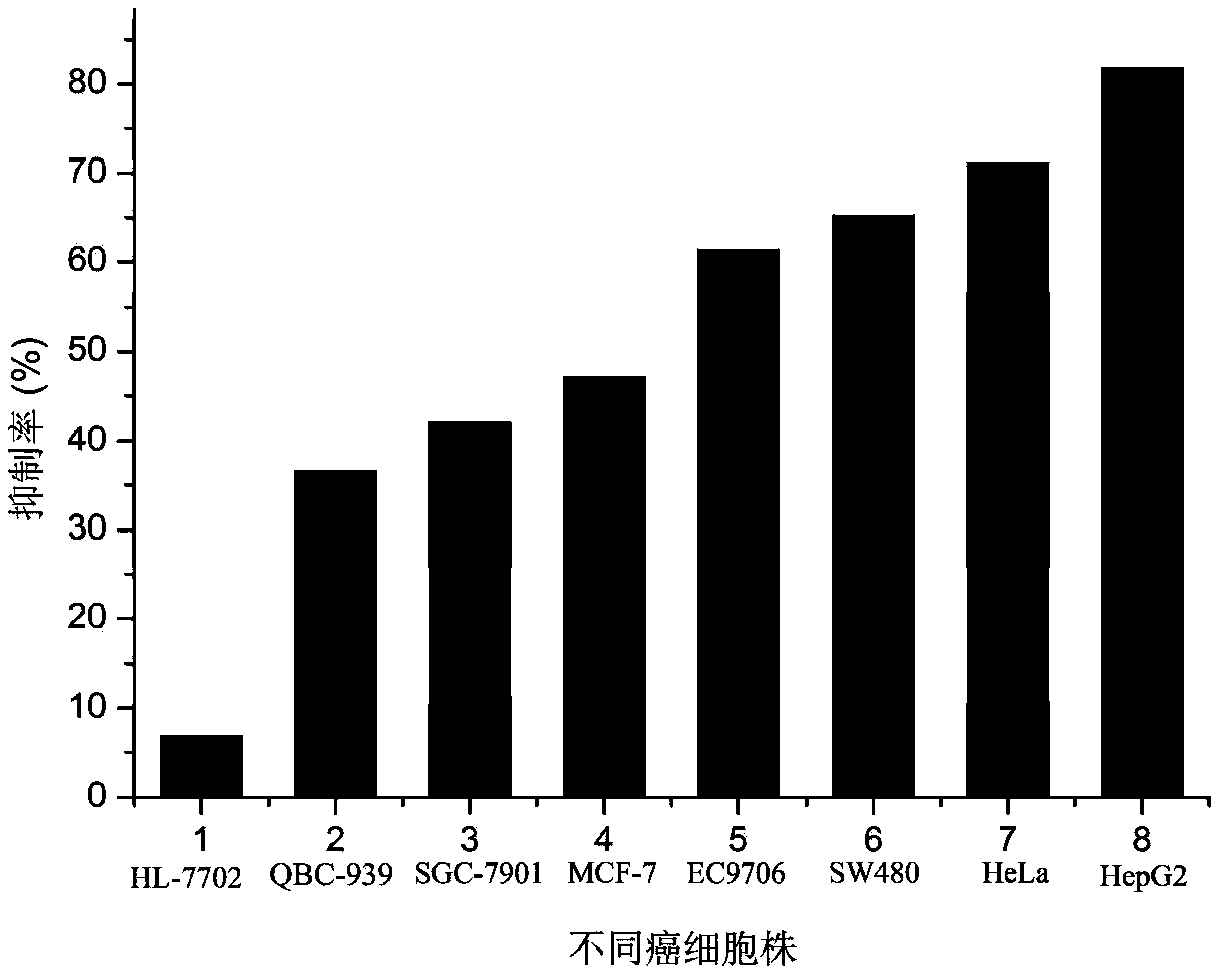
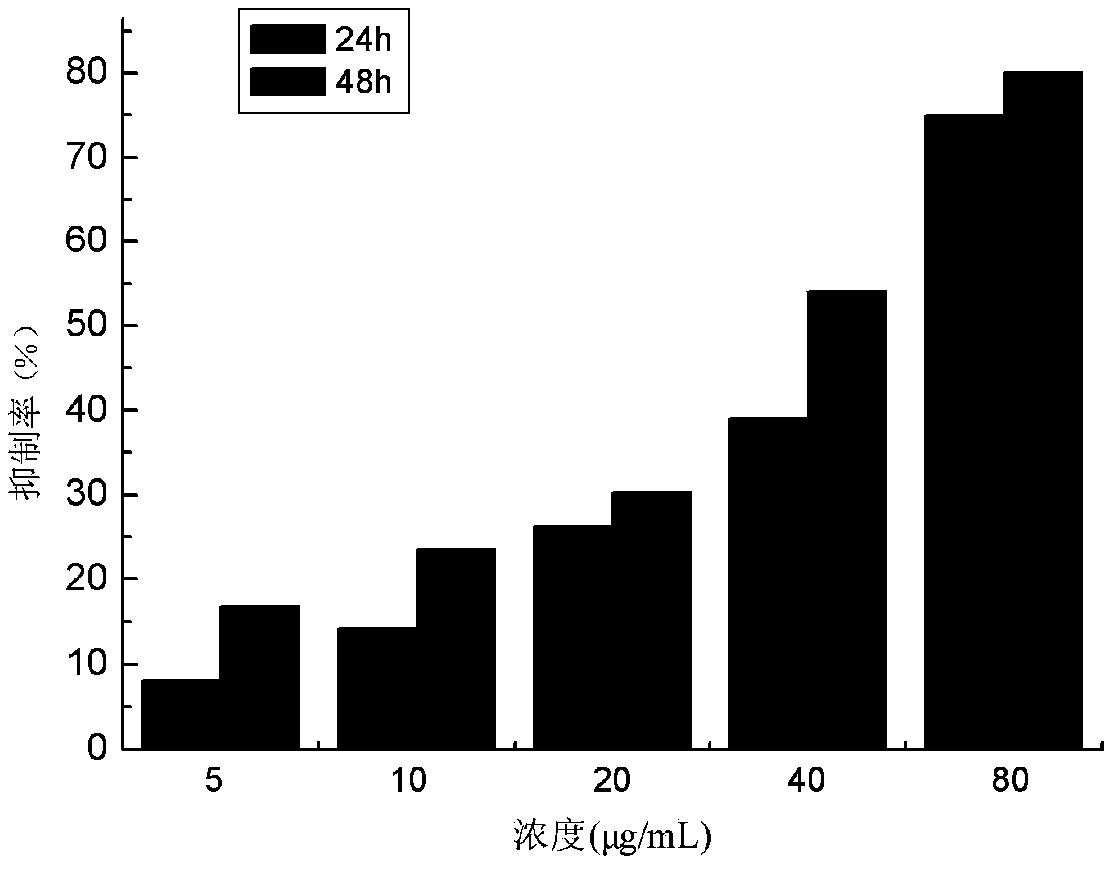
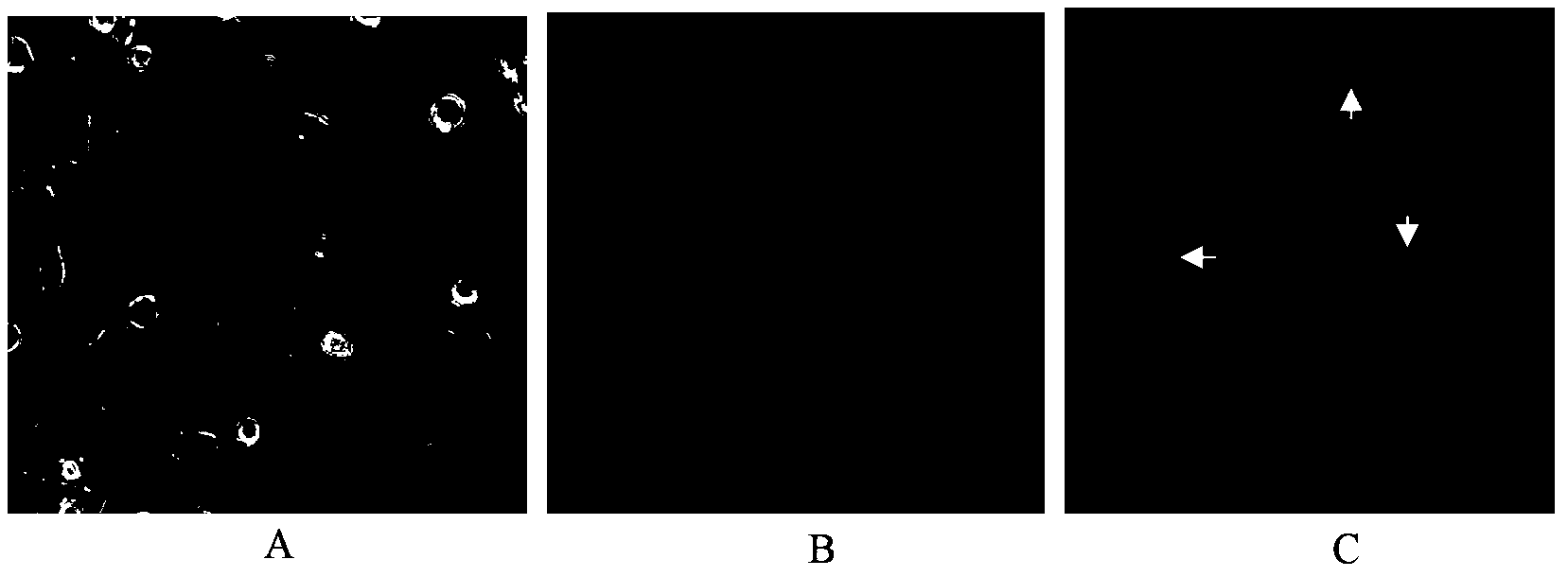
[0021] 1,4-Dimethyl-6,8-dimethoxy-9,10-anthraquinone in vitro anticancer activity experiment:
[0022] The anticancer activity experiment was realized by cytotoxicity, and the proliferation inhibitory activity of the compound on seven tumor cell lines was measured by standard MTT colorimetry. The tested tumor cell lines are human liver cancer cell line HepG2, gastric cancer cell line SGC-7901, breast cancer cell line MCF-7, cervical cancer cell line HeLa, bile duct cancer cell line QBC-939, esophageal cancer cell line EC9706, colon cancer cell line SW480 , while using human normal liver cells HL-7702 as a control.
[0023] The specific steps are: take the cells in the logarithmic growth phase and in good condition, add 0.25% trypsin to digest, make a single cell suspension, count on a cell counting plate, and inoculate 100 μL per well in a 96-well plate. The number of cells was controlled at 5×104 / mL, cultured overnight in a 5% CO2 incubator at 37°C, and prepared samples of d...
Embodiment 2
[0035] Example 2 Interaction between 1,4-dimethyl-6,8-dimethoxy-9,10-anthraquinone and bovine serum albumin
[0036] Absorption Spectroscopy Research
[0037] Figure 5 Curve 1 is the ultraviolet absorption spectrum of bovine serum albumin, and curve 2-10 is the ultraviolet absorption spectrum after adding different concentrations of the compound in bovine serum albumin. It can be seen from the figure that the absorption peak of bovine serum albumin after adding different concentrations of the compound Increased strength. It indicated that there was an interaction between the compound and BSA, and a complex with certain stability was formed.
[0038] Effects of Compounds on the Fluorescence Spectrum of Bovine Serum Albumin
[0039] Figure 6 Curve 1 is the fluorescence spectrum of bovine serum albumin, and curve 2-10 is the fluorescence spectrum after adding different concentrations of this compound in bovine serum albumin. It can be seen from the figure that the fluoresce...
Embodiment 3
[0040] Example 3 Interaction experiment between 1,4-dimethyl-6,8-dimethoxy-9,10-anthraquinone and DNA
[0041] Absorption Spectroscopy Research
[0042] Figure 7 Curve 1 is the ultraviolet absorption spectrum of DNA, and curves 2-9 are the ultraviolet absorption spectra of DNA after adding different concentrations of the compound. It can be seen from the figure that the DNA absorption peak intensity decreases after adding different concentrations of the compound. According to literature reports, when small molecules are intercalated between the base pairs of the CT-DNA double helix, the absorption spectrum exhibits a color reduction effect, and the wavelength is red-shifted; -DNA, its absorption spectrum shows no obvious color enhancement effect or color reduction, and no red shift; therefore, the interaction between the compound and CT-DNA at this time should be dominated by intercalation.
[0043] Compound Effects on DNA Fluorescence Spectrum
[0044] Figure 8 Curve 1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com