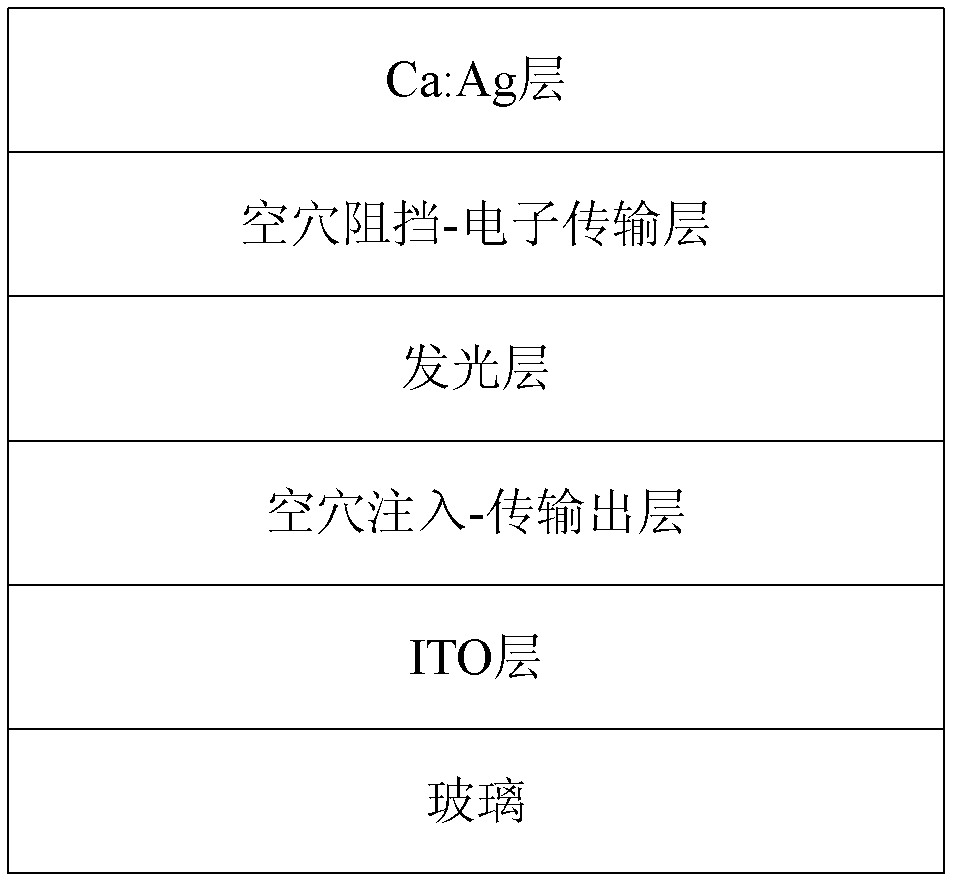
Cyano anthraquinone derivatives, preparation method and organic electroluminescent devices thereof
A cyano-anthraquinone and tetracyano-anthraquinone technology, applied in the field of organic optoelectronic materials, can solve the problems of inability to form high-quality thin films, device failure, rough surface, etc., and achieve good morphology stability and high singlet energy. The effect of grade and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
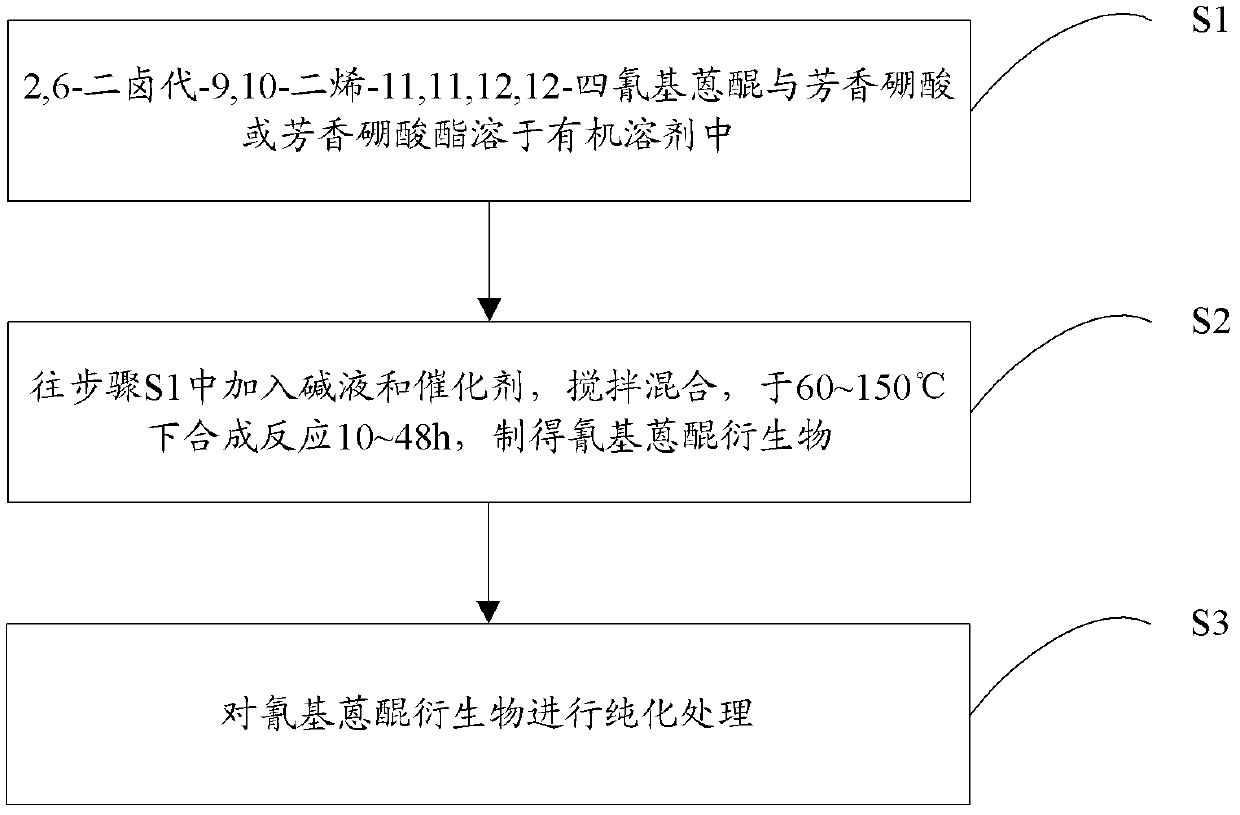
[0021] Another object of the present invention is to provide a preparation method of cyanoanthraquinone derivatives, comprising the following steps:
[0022] Step S1, the structural formula is The 2,6-dihalo-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the structural formula is The aromatic boronic acid or structural formula is The aromatic borate is dissolved in an organic solvent; where Ar is an aromatic group; X is Cl or Br; 2,6-dihalo-9,10-diene-11,11,12,12-tetracyano The molar ratio of base anthraquinone to aromatic boric acid or aromatic borate is 1:2-1:4;
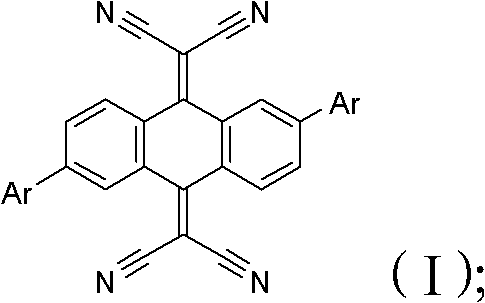
[0023] Step S2, add lye and catalyst to step S1, stir and mix, and synthesize and react at 60-130°C for 10-48 hours to obtain the cyanoanthraquinone derivative of the following general formula (I):
[0024]
[0025] The reaction formula is as follows:
[0026]
[0027] Further optimization of the above preparation method, that is, the crude product of the cyanoanthraquinone derivative obtained in ...
Embodiment 1
[0041] This example discloses 2,6-dipyrene-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the following structural formula.
[0042]
[0043] The preparation of the above-mentioned target molecule is as follows:
[0044] Under the protection of nitrogen, 2,6-dibromo-9,10-diene-11,11,12,12-tetracyanoanthraquinone (4.15g, 9mmol), pyreneboronic acid (5.76g, 23.4mmol) and four Triphenylphosphine palladium (104mg, 0.09mmol) was dissolved in 80ml of ethylene glycol dimethyl ether, then sodium carbonate solution (2.0mol / L, 90mL) was added and mixed; the mixture was vigorously stirred at 90°C for 12h. Cool to room temperature, extract three times with dichloromethane, combine the organic phases, wash with 1mol / L sodium hydroxide solution, spin dry after drying over anhydrous magnesium sulfate, use petroleum ether:ethyl acetate (10:1) as the crude product The eluent was separated by silica gel chromatography to obtain a white solid, namely 2,6-dipyrene-9,10-diene-11,11,12,12-t...
Embodiment 2
[0047] This example discloses 2,6-bis[10'-(9'-phenyl)anthracene]-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the following structural formula.
[0048]
[0049] The preparation of the above-mentioned target molecule is as follows:
[0050] Under nitrogen protection, 2,6-dibromo-9,10-diene-11,11,12,12-tetracyanoanthraquinone (4.15g, 9mmol), 9-phenyl-10-anthraceneboronic acid (5.76 g, 23.4mmol) and bistriphenylphosphine palladium dichloride (315.9mg, 0.45mmol) were dissolved in 80ml of tetrahydrofuran, and then sodium carbonate solution (2.0mol / L, 90mL) was added. The mixture was vigorously stirred at 90°C for 18h. Cool to room temperature, extract three times with dichloromethane, combine the organic phases, wash with 1mol / L sodium hydroxide solution, spin dry after drying over anhydrous magnesium sulfate, use petroleum ether:ethyl acetate (10:1) as the crude product The eluent was separated by silica gel chromatography to obtain a white solid, namely 2,6-bis[10'-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com