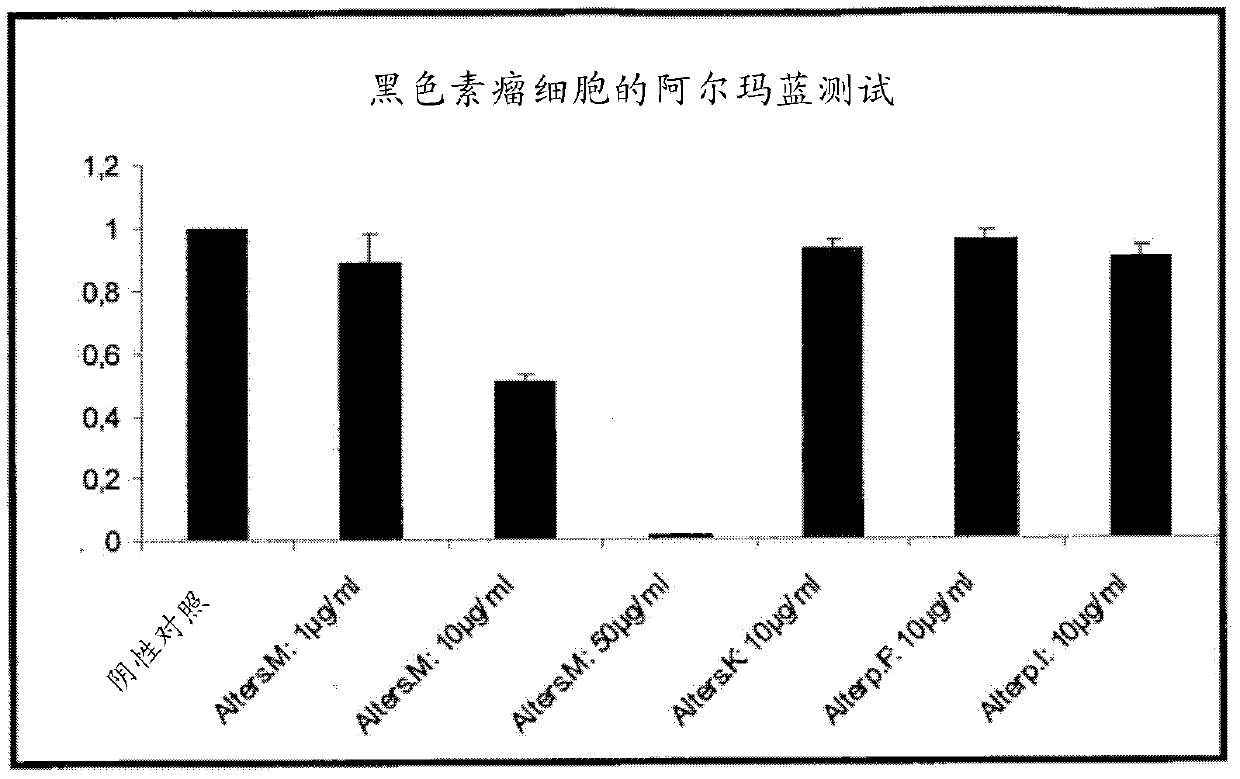
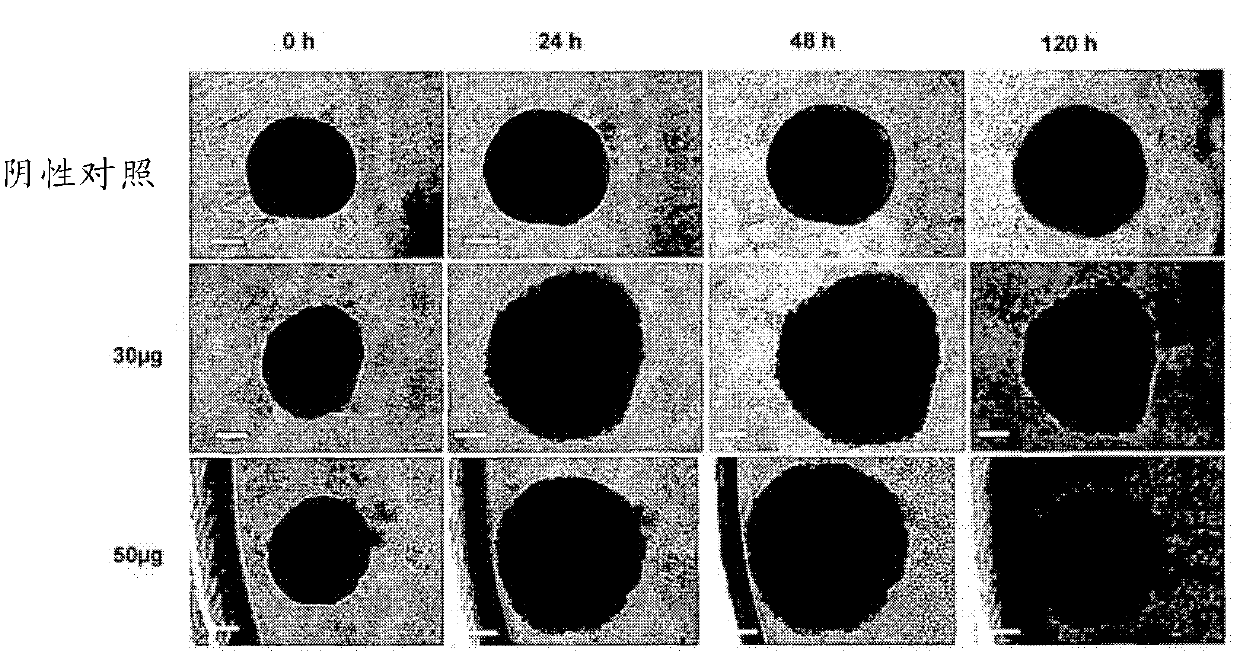
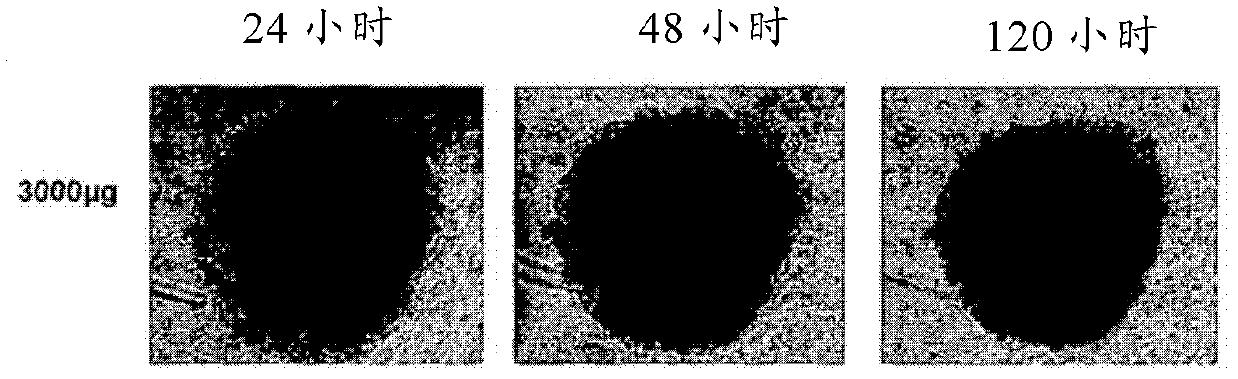
Novel anthraquinone derivatives
An anthracene-based, compound technology, applied in the field of new anthraquinone derivatives, can solve the problems of outflow, no longer effective, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0068] Hereinafter, the present invention is described in further detail with reference to specific exemplary embodiments, however, this is not meant to limit the scope of the present invention in any way.
[0069] The novel compounds of the present invention are obtained by culturing Puccinia glomerulus and subsequent extraction.
[0070] isolate microorganism
[0071] Endophytic fungi were isolated from fresh, healthy stems of Mint lipelum (Peppermint). The surface of the stem was sterilized with 70% ethanol for 1 min, followed by rinsing with sterile water to remove the ethanol. To distinguish endophytic fungi from any remaining epiphytic fungi, stem surface imprints were made on organic malt extract agar. A small sample of internal tissue is sterilely sectioned and pressed onto agar plates containing an antibiotic to inhibit bacterial growth. The composition of the isolation medium was as follows: 15 g / l malt extract, 15 g / l agar, and 0.2 g / l chloramphenicol in distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com