Preparation method of anthraquinone-molecule non-covalent modified graphene/conductive polymer composite
A conductive polymer and non-covalent modification technology, which is applied in the manufacture of hybrid/electric double layer capacitors, hybrid capacitor electrodes, etc., can solve the problems of small effective specific surface area of composite materials, and achieve easy scale-up production, wide application prospects, The effect of increasing energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
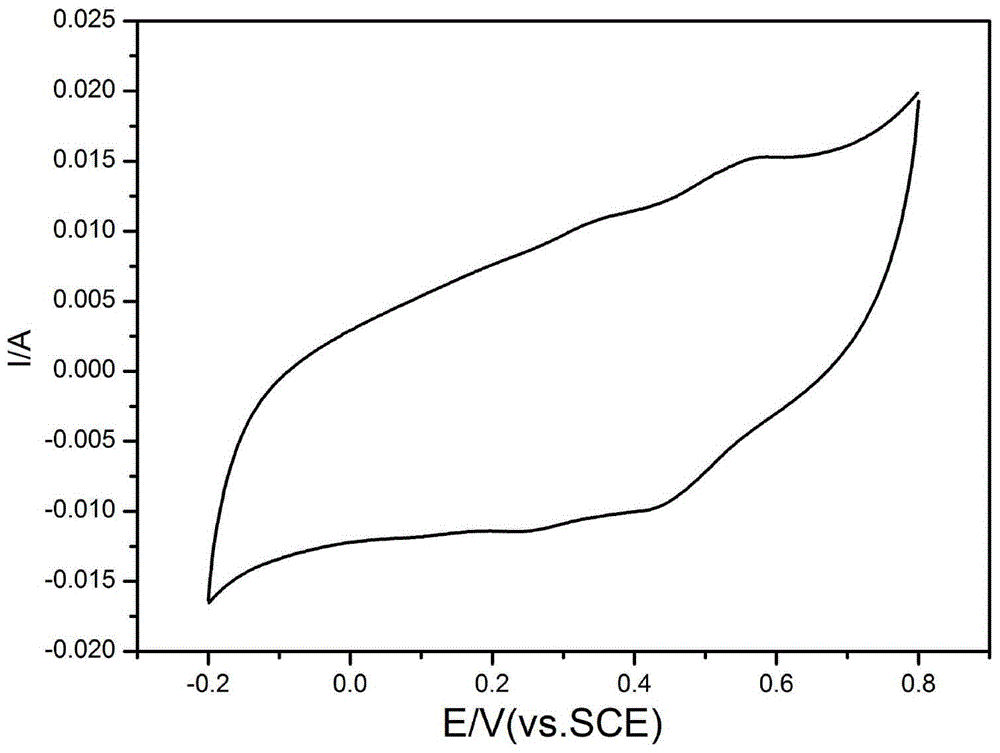
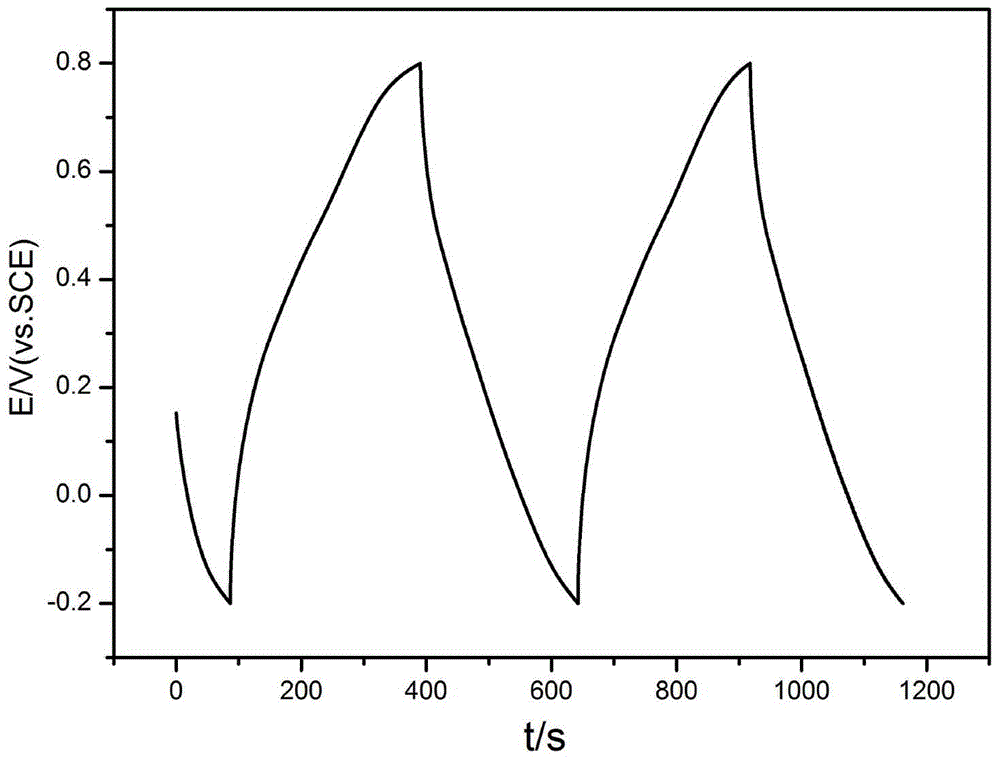
Image
Examples
Embodiment 1
[0020] Embodiment 1 A preparation method of anthraquinone molecule non-covalently modified graphene / conductive polymer composite material, the steps are as follows:
[0021] (1) Dissolve 1.5513g (5mmol) sodium anthraquinone-1-sulfonate (purchased from Shanghai Future Industrial Co., Ltd.) in 100mL 0.01mg / mL graphene oxide solution (for the preparation method refer to Hummers WS, Offeman R E. Preparation of graphite oxide. J Am Chem Soc, 1958, 80: 1339)), stirred and dispersed by ultrasonic waves to form a solution, set aside;
[0022] (2) Add 67 μL (1 mmol) of pyrrole monomer (purchased from Aldrich) to the above dispersion, stir and disperse with ultrasonic waves to form a reaction system, set aside;
[0023] (3) Stir the above reaction system at 30°C for 48 hours to obtain the product;
[0024] (4) The obtained product was repeatedly washed with deionized water, and dried in a vacuum drying oven at 60°C for 24 hours to obtain a non-covalently modified graphene / conductive po...
Embodiment 2
[0025] Embodiment 2. A preparation method of anthraquinone molecule non-covalently modified graphene / conductive polymer composite material, which is different from Example 1 in that the concentration of graphene oxide becomes 0.4mg / mL, 1.5513g (5mmol) anthraquinone- 1-sodium sulfonate is changed to 4.6539g (15mmol) sodium anthraquinone-2-sulfonate, 67μL (1mmol) pyrrole monomer is changed to 450μL (5mmol) aniline monomer, stirring reaction at 30°C for 48h becomes 60°C reaction 36h.
Embodiment 3
[0026] Embodiment 3. A preparation method of anthraquinone molecule non-covalently modified graphene / conductive polymer composite material, which differs from Example 1 in that the concentration of graphene oxide becomes 0.2mg / mL, 1.5513g (5mmol) anthraquinone- Sodium 1-sulfonate becomes 1.6655g (4mmol) of acid blue 25, 67μL (1mmol) of pyrrole monomer becomes 220μL (2mmol) of 3,4-ethylenedioxythiophene, and the stirring reaction is carried out at 30°C for 48h to 90°C Reaction 24h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com