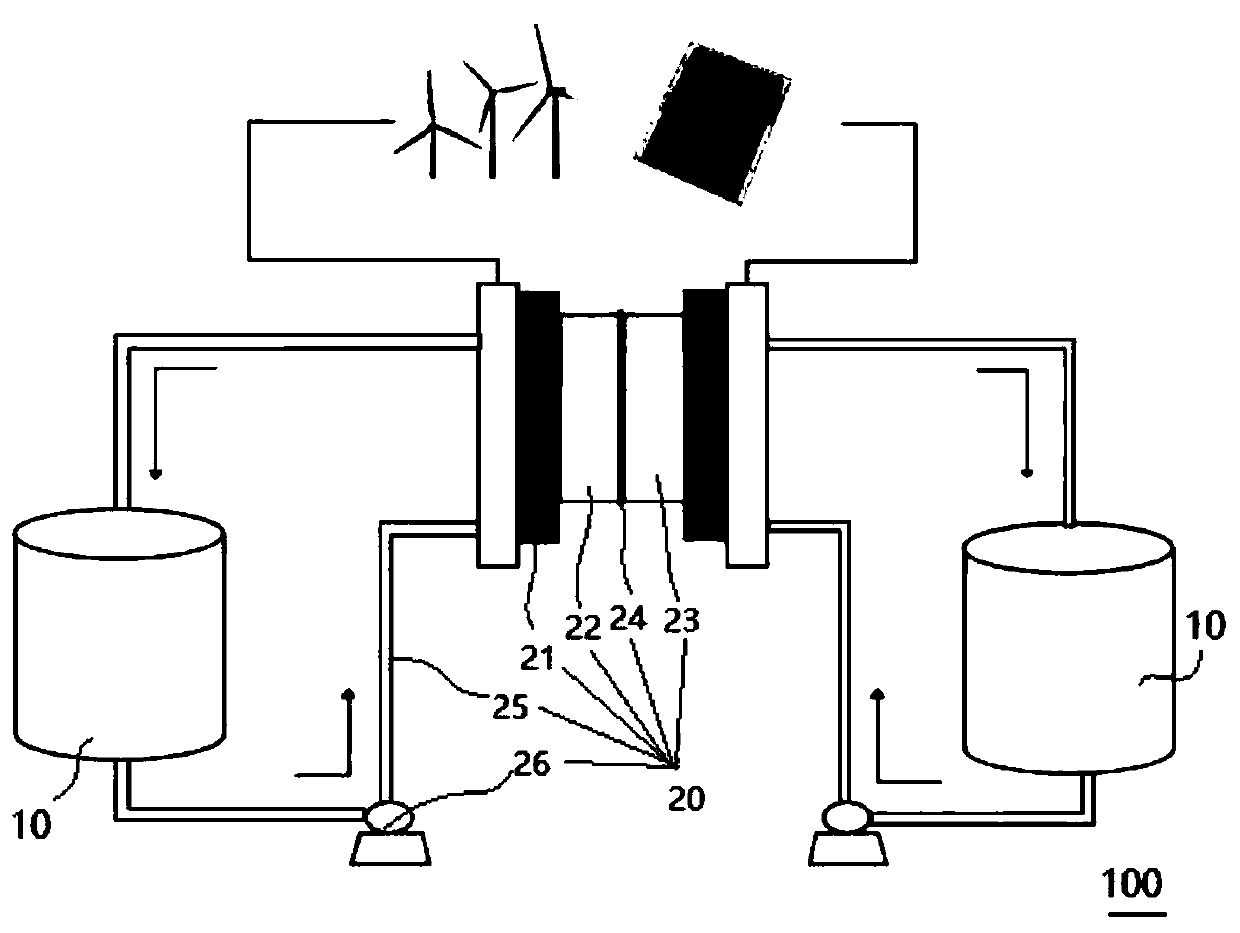
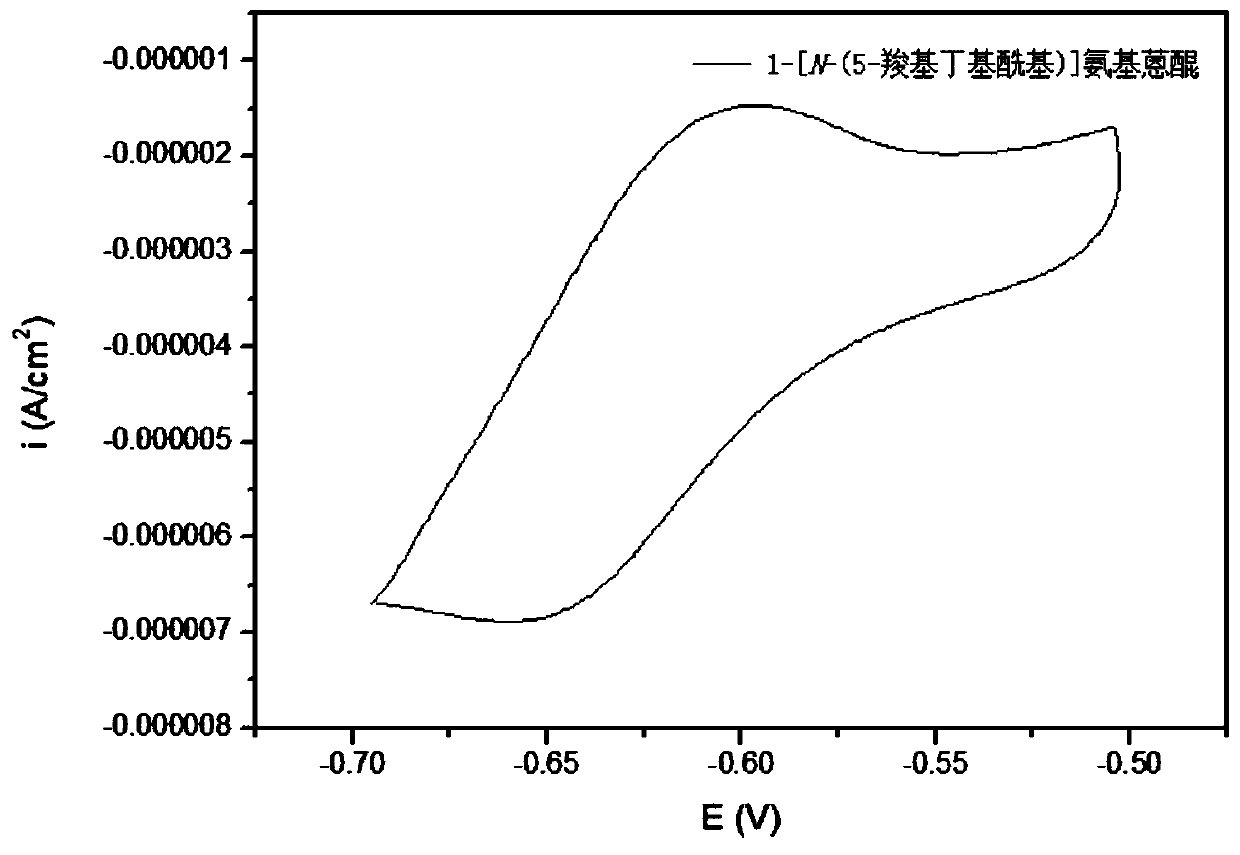
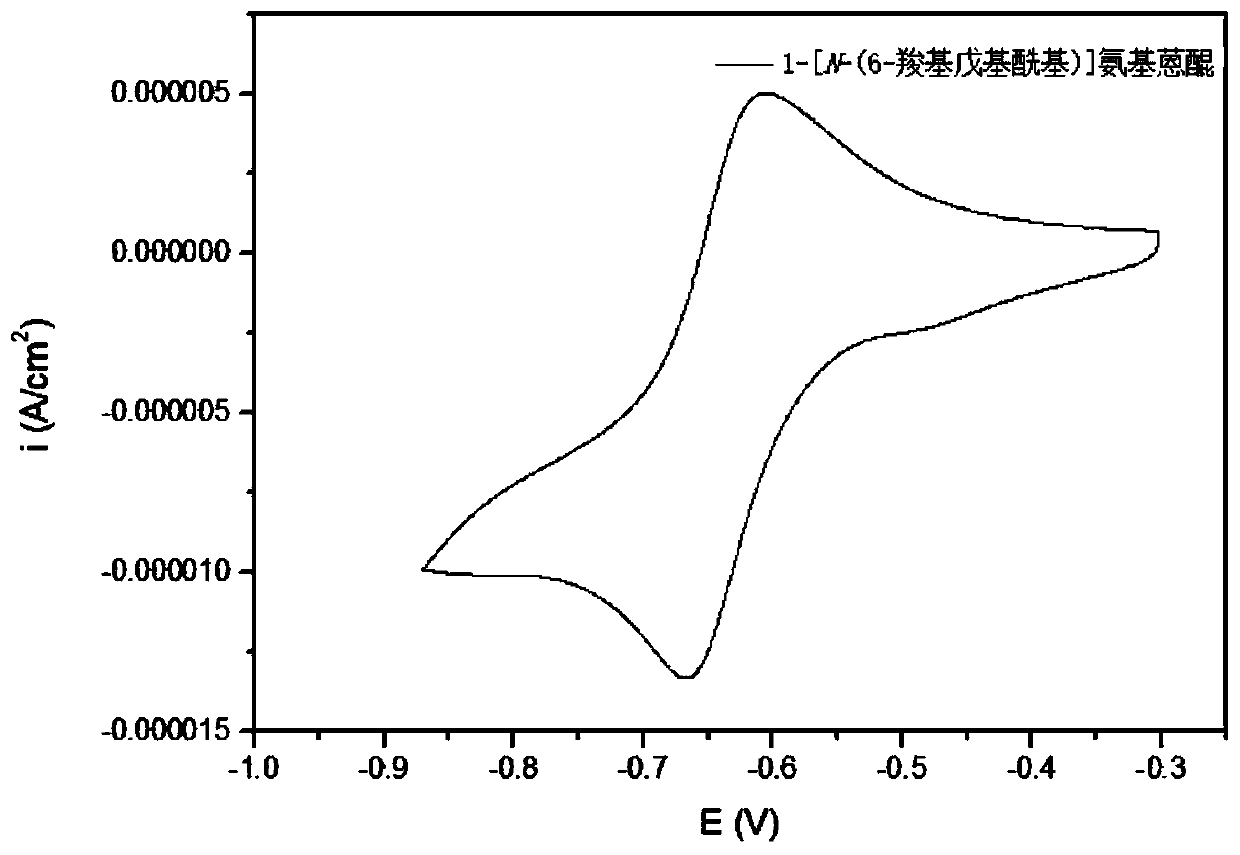
Synthesis method, derivative and battery system of anthraquinone derivative containing carboxyl group
A technology of anthraquinone derivatives and synthesis methods, which is applied in the field of liquid flow batteries, can solve problems such as prone to water electrolysis side reactions, limited solubility of active materials, and easy cross-contamination of electrolytes, and achieve low cost, high solubility, and safety performance high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The synthesis method of the carboxyl-containing anthraquinone derivative according to the embodiment of the present invention comprises the following steps:
[0052] S1. Mix the carboxyl-terminated dibasic acid with thionyl chloride and add toluene as a reaction solvent, add a catalyst, and heat to a set temperature for reaction;
[0053] S2, after the reaction finishes, remove solvent and thionyl chloride, add toluene to distill, obtain reactant;
[0054] S3. Mix the reactant with aminoanthraquinone, add toluene as a reaction solvent, and heat up to reflux for reaction;
[0055] S4. After the reaction, the solvent is removed, potassium carbonate solution is added to the residue, the solid is removed by filtration, the pH of the filtrate is adjusted to a predetermined value, the solid is precipitated, filtered, washed and dried to obtain an anthraquinone derivative containing carboxyl groups.
[0056] Specifically, at first the acid chlorination of the carboxyl-termina...
Embodiment 1
[0094] Synthesis of 1-[N-(6-carboxypentyl)]aminoanthraquinone
[0095] 2.92g of adipic acid (0.02mol) and 15mL of thionyl chloride were mixed and dissolved in 35mL of toluene, and 0.01g of DMF was added as a catalyst. The temperature was raised to 60°C and the reaction was refluxed, and the reaction was stopped when the solvent was light yellow (12h-24h). Thionyl chloride and toluene were distilled off under reduced pressure, and toluene was added for distillation (20 mL×2), and the residue was used for the following reaction.
[0096] Add 40 mL of toluene and 0.89 g of 1-aminoanthraquinone to the above residue in sequence, and slowly raise the temperature to reflux. As the reaction progressed, the reaction solution gradually changed from red to orange-yellow. The progress of the reaction was monitored by TLC and the reaction was stopped when the reaction was almost complete (15h-20h). The solvent toluene was distilled off under reduced pressure (to be completely steamed as...
Embodiment 2
[0098] Synthesis of 1-[N-(8-carboxyheptyl)]aminoanthraquinone
[0099] 3.48g of suberic acid (0.02mol) and 15mL of thionyl chloride were mixed and dissolved in 35mL of toluene, and 0.01g of pyridine was added as a catalyst. The temperature was raised to 60°C and the reaction was refluxed, and the reaction was stopped when the solvent was light yellow (12h-24h). Thionyl chloride and toluene were distilled off under reduced pressure, and toluene was added for distillation (20 mL×2), and the residue was used for the following reaction.
[0100] Add 40 mL of toluene and 0.89 g of 1-aminoanthraquinone to the above residue in sequence, and slowly raise the temperature to reflux. As the reaction progressed, the reaction solution gradually changed from red to orange-yellow. The progress of the reaction was monitored by TLC and the reaction was stopped when the reaction was almost complete (15h-20h). The solvent toluene was distilled off under reduced pressure (to be steamed out com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com