Pharmaceutical composition of cortex fraxini extract and borneol as well as preparation method and application of pharmaceutical composition
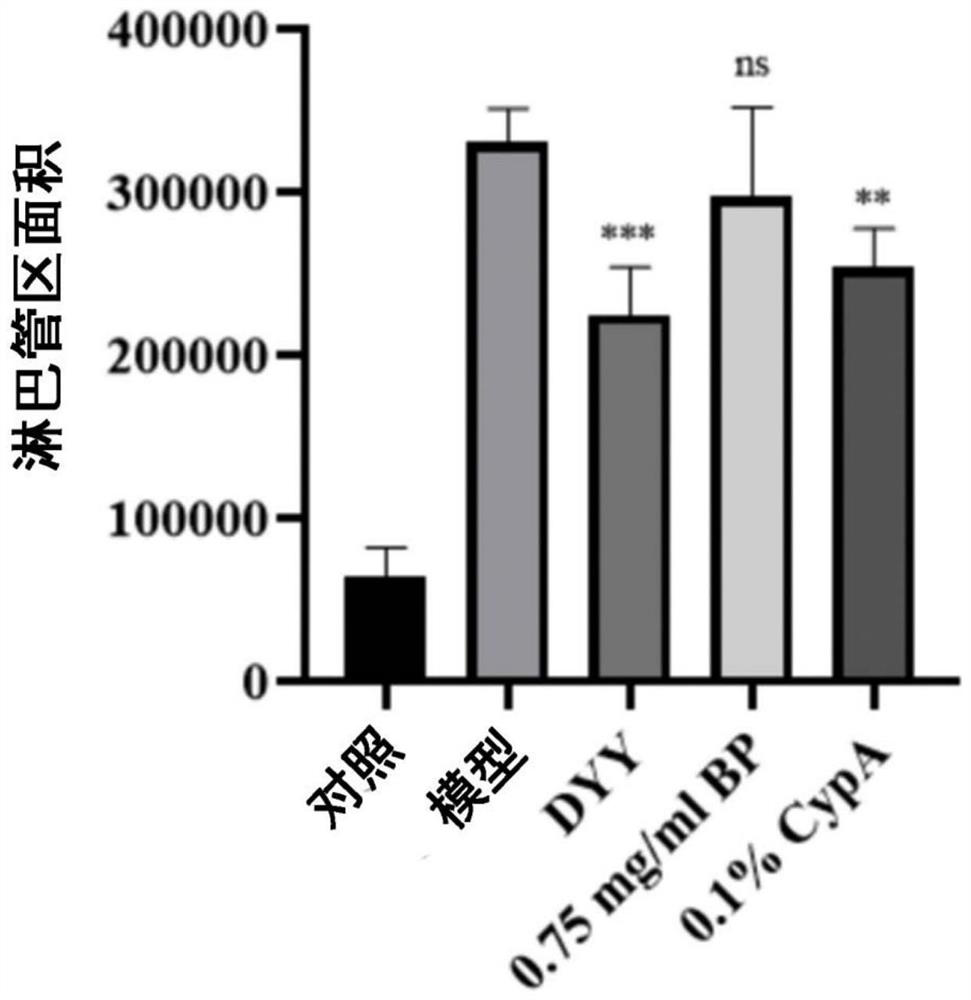
A technology of composition and extract, which is applied in the direction of drug combination, pharmaceutical formula, active ingredients of hydroxyl compounds, etc., can solve the problems such as the development and efficacy evaluation of the total coumarin of phoenix bark, and achieve good therapeutic effect and improve inhibition The effect of inhibiting the formation of new lymphatic vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Static adsorption method screening macroporous resin
[0067] Take by weighing 100g of Cortex chinensis medicinal material, add 8 times the amount (800ml) of 75% ethanol and heat and reflux to extract twice, each time for 2 hours, the extract is recovered under reduced pressure to ethanol and concentrated to 133ml, which is the Pitt officinalis extract.
[0068] 25g of different types of macroporous resins (D101, D301R, HPD100, ADS-7, HD450, HPD500) were used for static adsorption with 10ml of Qinpi extract, and the adsorption rate was calculated.
[0069] Adsorption rate (%)=(total coumarin concentration in stock solution-total coumarin concentration in filtrate after adsorption)×filtrate volume / total coumarin content in stock solution.
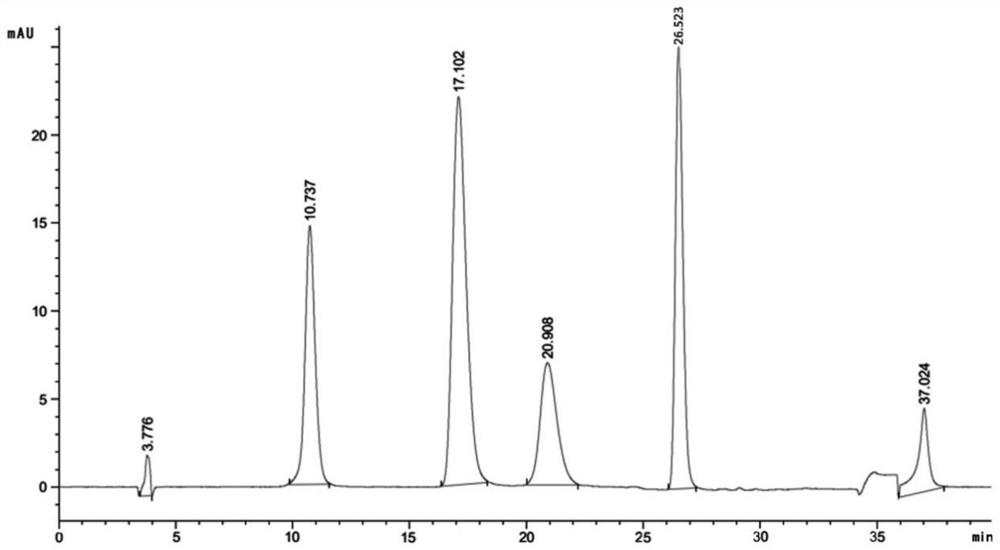
[0070] The results showed that: D101, D301R, HPD100, ADS-7, HD450, and HPD500 above seven kinds of macroporous resins had higher than 50% adsorption rates of aureticin, aureticin, aureticin and aureticin, among which D301...
Embodiment 2
[0071] The preparation of embodiment 2 Cortex chinensis extract
[0072] Weigh 100g of Pittia chinensis medicinal material, add 8 times the amount (800ml) of 75% ethanol and heat and reflux to extract twice, each time for 2 hours, the extract is recovered under reduced pressure to recover ethanol and concentrated to 133ml (0.75g crude drug / ml). The sample loading volume is 0.8 times the column volume (BV), and the treated ADS-7 macroporous resin (washed with 95% ethanol until there is no turbidity, and then washed with distilled water until there is no alcohol smell) at a volume flow rate of 2BV / h, 2 times The column volume was eluted with water to remove impurities, and then eluted with 6 times the column volume of 25% ethanol, discarding the first 1 times the column volume eluate, collecting the 2-6 times the column volume of the 25% ethanol eluate, and reducing pressure Concentrate and dry to obtain the extract of bark bark.
[0073] Determination of active ingredients in ...
Embodiment 3
[0089] The preparation of embodiment 3 Qin bark extract
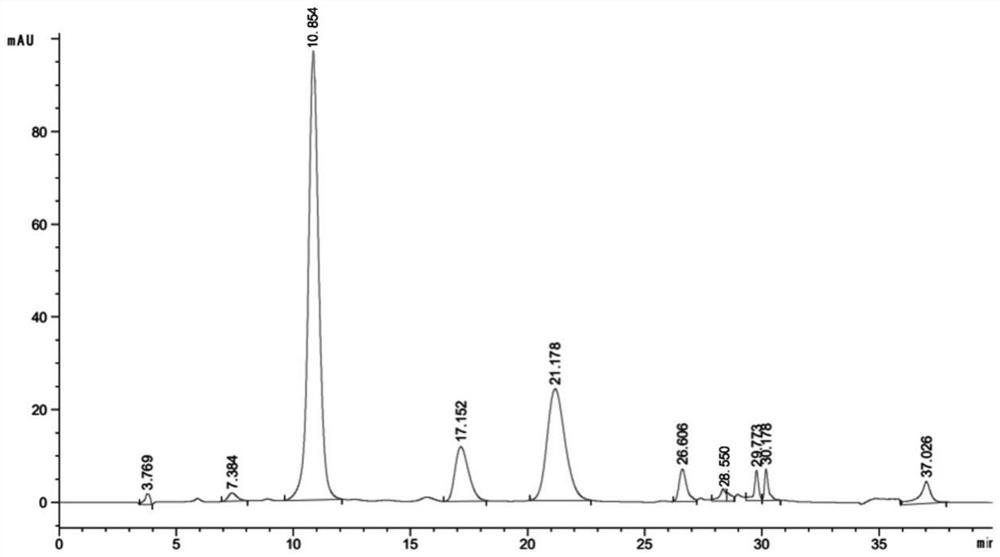
[0090] Weigh 100g of the medicinal material of Cortex chinensis, add 8 times the amount of 75% ethanol and heat and reflux to extract twice, each time for 2 hours, the extract is recovered under reduced pressure to recover ethanol and concentrated to 133ml (0.75g crude drug / ml). The sample loading volume is 0.8 times the column volume (BV), and the treated D301R macroporous resin is passed through the treated D301R macroporous resin at a volume flow rate of 2 BV / h, and impurities are eluted with 2 times the column volume of water, and then 7 times the column volume of 30% ethanol is used for elution , Discard the first 1 column volume eluate, collect the 30% ethanol eluate of the 3rd to 7th column volume, concentrate and dry under reduced pressure to obtain the extract of Pittia chinensis.
[0091] Measured according to the method of the above-mentioned embodiment 2, the total coumarin content in the bark extract is 77.6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com