Application of umbilical cord mesenchymal stem cells to preparation of stem cell preparation for treating corneal injury
A stem cell preparation and quality stem cell technology, applied in the application field of stem cell preparations, can solve the problems such as the cornea cannot be restored to transparency, the rejection is severe, and the curative effect is poor, and achieves effective repair of corneal epithelial tissue, weak immunogenicity, and recovery. The effect of integrity and transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of umbilical cord-derived mesenchymal stem cell preparation
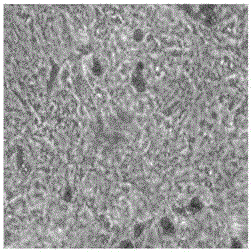
[0038] Operate in an ultra-clean workbench, sterilize the freshly obtained umbilical cord with ethanol, wash the umbilical cord with normal saline, separate and strip off the umbilical cord blood vessels, obtain the Wharton's jelly part, cut the Wharton's jelly into pieces, dilute it, and plant it in a bottle. Cultured in a carbon dioxide incubator using the tissue-attachment method. Observe the growth of the cells under a microscope. It can be seen that the cells are attached to the wall and grow in the shape of a long spindle. The cytoplasm is clear, the cell body is small, and has a three-dimensional effect. For non-adherent cells, the primary cells can be harvested when the cell fusion degree reaches 70-80%; the obtained primary cells are subcultured in DMEM medium to achieve the purpose of purifying and expanding the culture of mesenchymal stem cells, the results See figure 1 .
Embodiment 2
[0039] Example 2: Research on surface markers of umbilical cord-derived mesenchymal stem cells
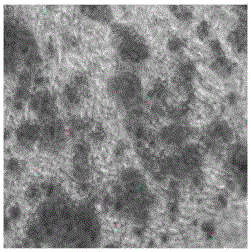
[0040] The surface markers of the second-generation umbilical cord mesenchymal stem cells obtained in Example 1 were detected by flow cytometry, and the results are shown in Table 1 and figure 2 . Cell surface markers CD73, CD90, and CD105 related to MSCs were highly expressed, and the expressions of each generation were stable, all higher than 95%; cell surface markers related to hematopoietic cells CD11b, CD34, CD45, CD19 and immune rejection related The expression of HLA-DR was low, all less than 3%. It has been proved that MSCs derived from umbilical cord after multiple passages contain stem cells that can stably proliferate. The cells highly express MSCs-related markers, and do not express or low-express hematopoietic cells and cell surface markers related to transplant rejection, suggesting that this type of MSCs is a kind of low-grade MSCs. Immunogenic stem cells.
[004...
Embodiment 3
[0043] Example 3: Study on proliferation of umbilical cord-derived mesenchymal stem cells
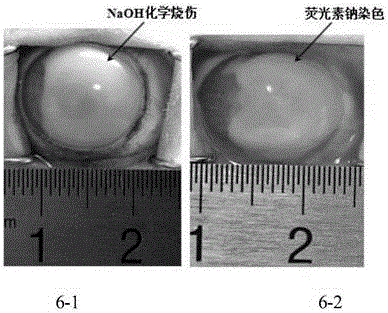
[0044] Take P5 generation UC-MSC and inoculate in 24-well plate, 1×10 4 cells / hole. 3 wells were randomly collected and counted every day for 7 consecutive days to make growth curves. The results are shown in Table 2 and image 3 . The results showed that the growth incubation period was 0-4 days after inoculation of the P5 generation cells; about 4-7 days, the cell growth was active, which was a logarithmic growth period; after that, the cell growth entered the plateau period, and the cell growth curve was S-shaped.
[0045] Table 2. UC-MSCs cell count results
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com