Patents
Literature
114 results about "Xenograft Transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Xenotransplantation (xenos- from the Greek meaning "foreign"), is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants.
Anticoagulant fusion protein anchored to cell membrane
InactiveUS6423316B1Prolong clotting timeGood curative effectFungiVirusesCell membraneBlood coagulations
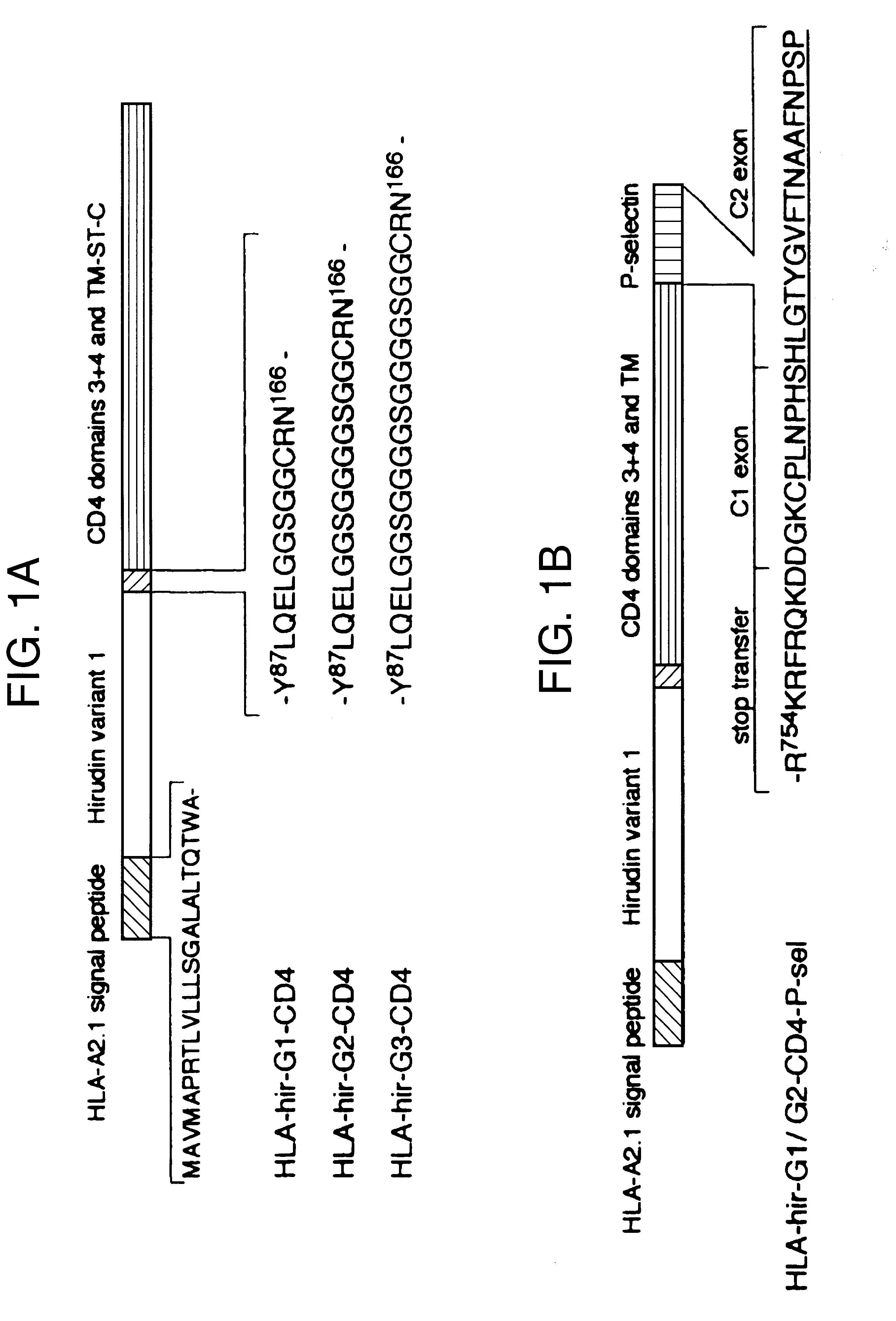
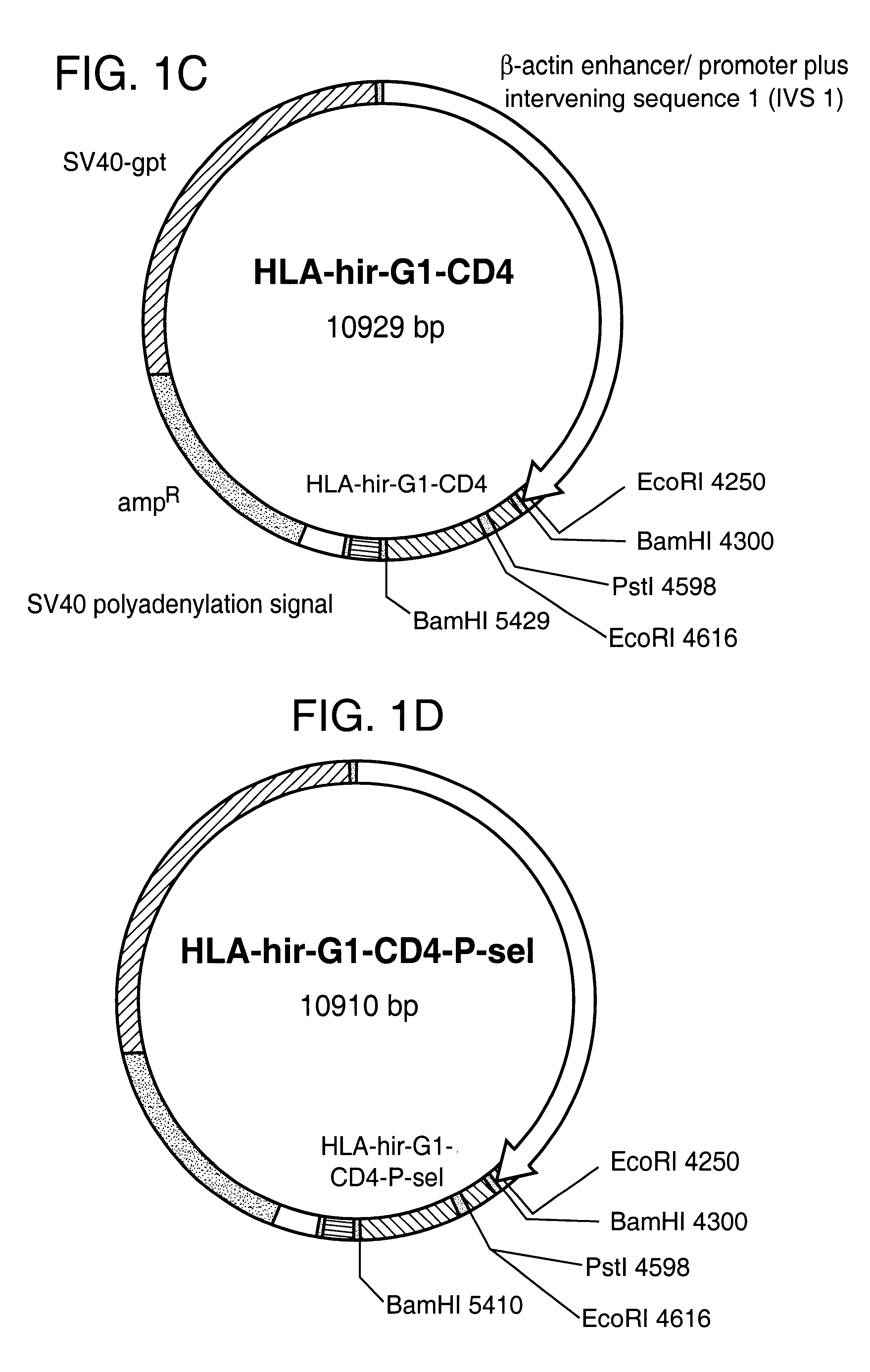
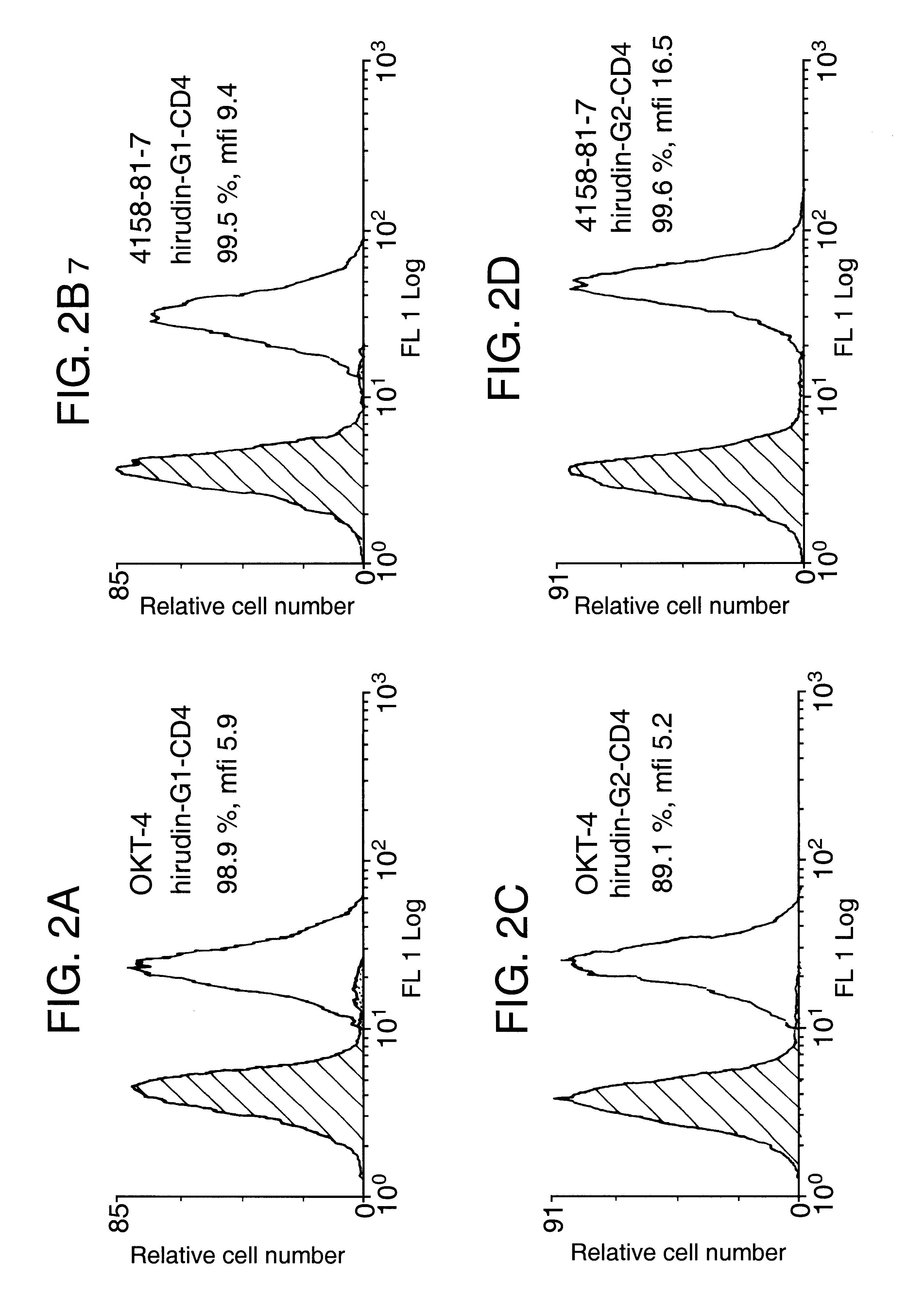
The invention relates to the inhibition of blood coagulation, especially during organ rejection, and in particular the inhibition of delayed vascular rejection. The invention provides anticoagulant proteins which are anchored to cell membranes. The anticoagulant function preferably provided by heparin, antithrombin, hirudin, TFPI, tick anticoagulant peptide, or a snake venom factor. These anticoagulant proteins are preferably prevented from being constitutively expressed at the cell surface. In particular, expression at the cell surface is regulated according to cell activation, for instance by targeting the protein to a suitable secretory granule. Expression of these proteins renders cells, tissues and organs less vulnerable to rejection after transplantation (e.g. after xenotransplantation).
Owner:IMPERIAL INNOVATIONS LTD
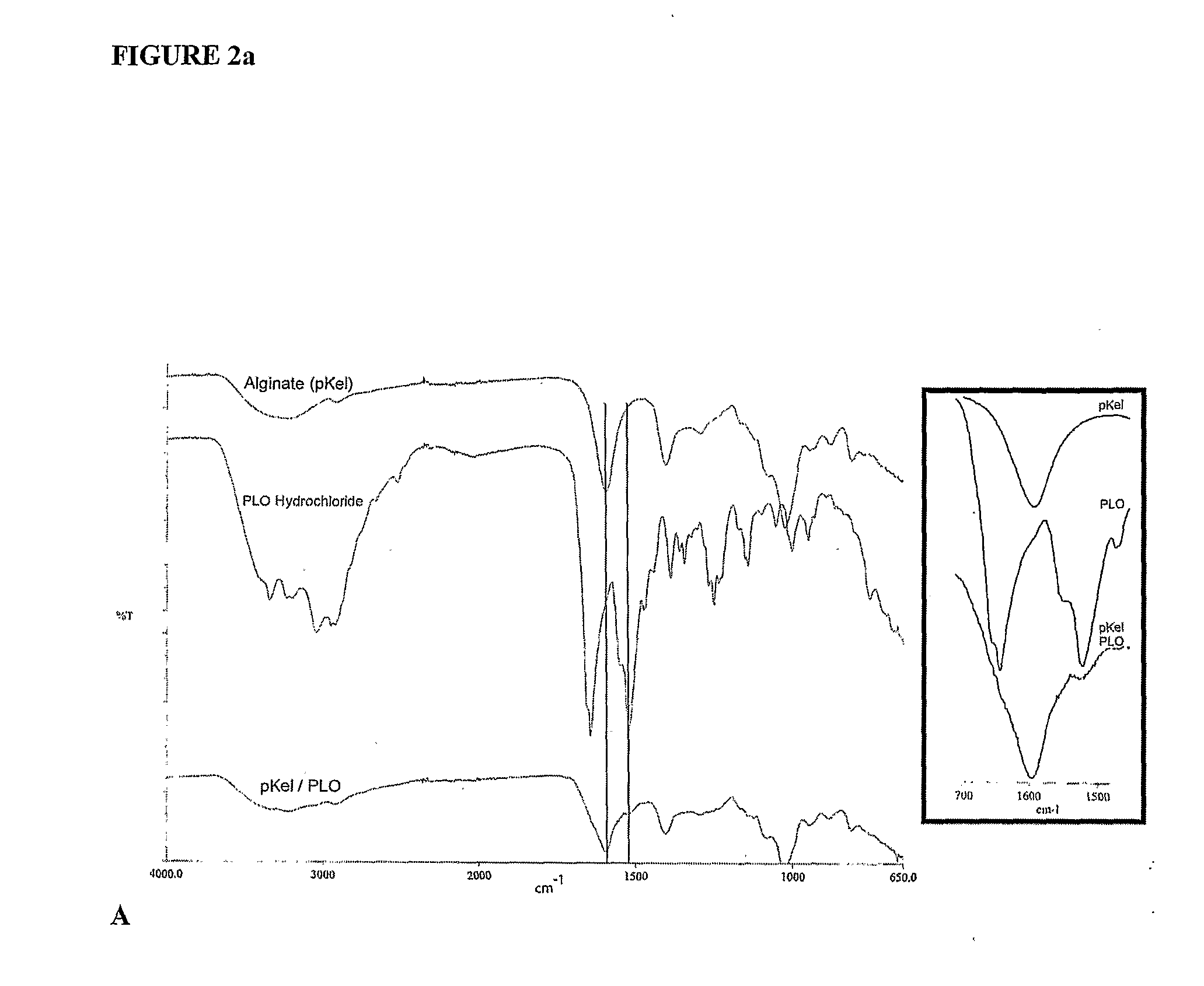
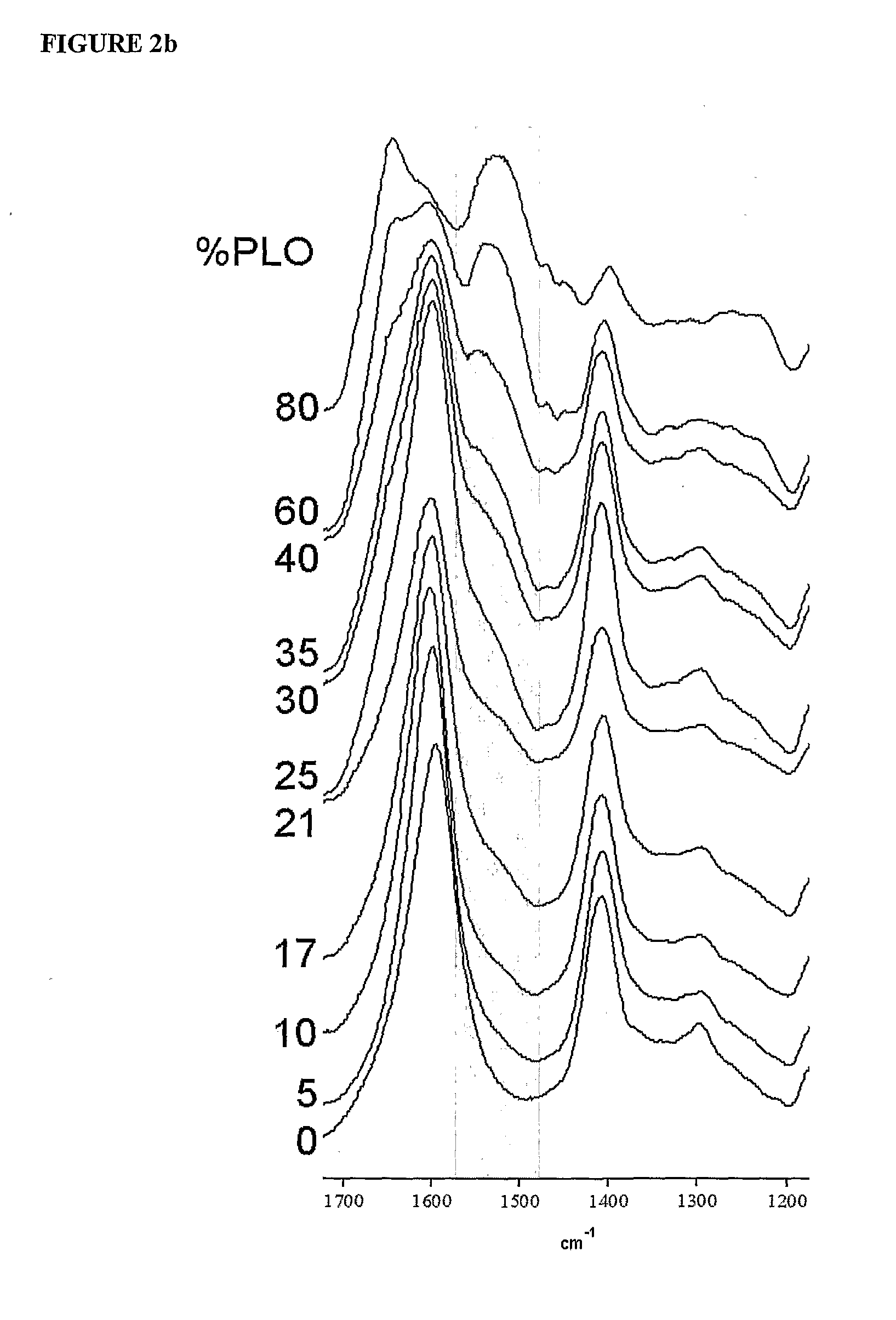
Encapsulation system
InactiveUS20090214660A1Improve protectionReduce the degradation rateBiocideNervous disorderMedicineFunctional integrity
The present invention is directed to a composition comprising high mannuronic acid-containing alginate and a polycation having a polydispersity index of less than 1.5. The composition is particularly useful for making biocompatible microcapsules containing living cells for allo- or xeno-transplantation. Such microcapsules have enhanced durability and can maintain their structural and functional integrity over long periods of time compared to prior art alginate microcapsules.
Owner:LIVING CELL PRODS
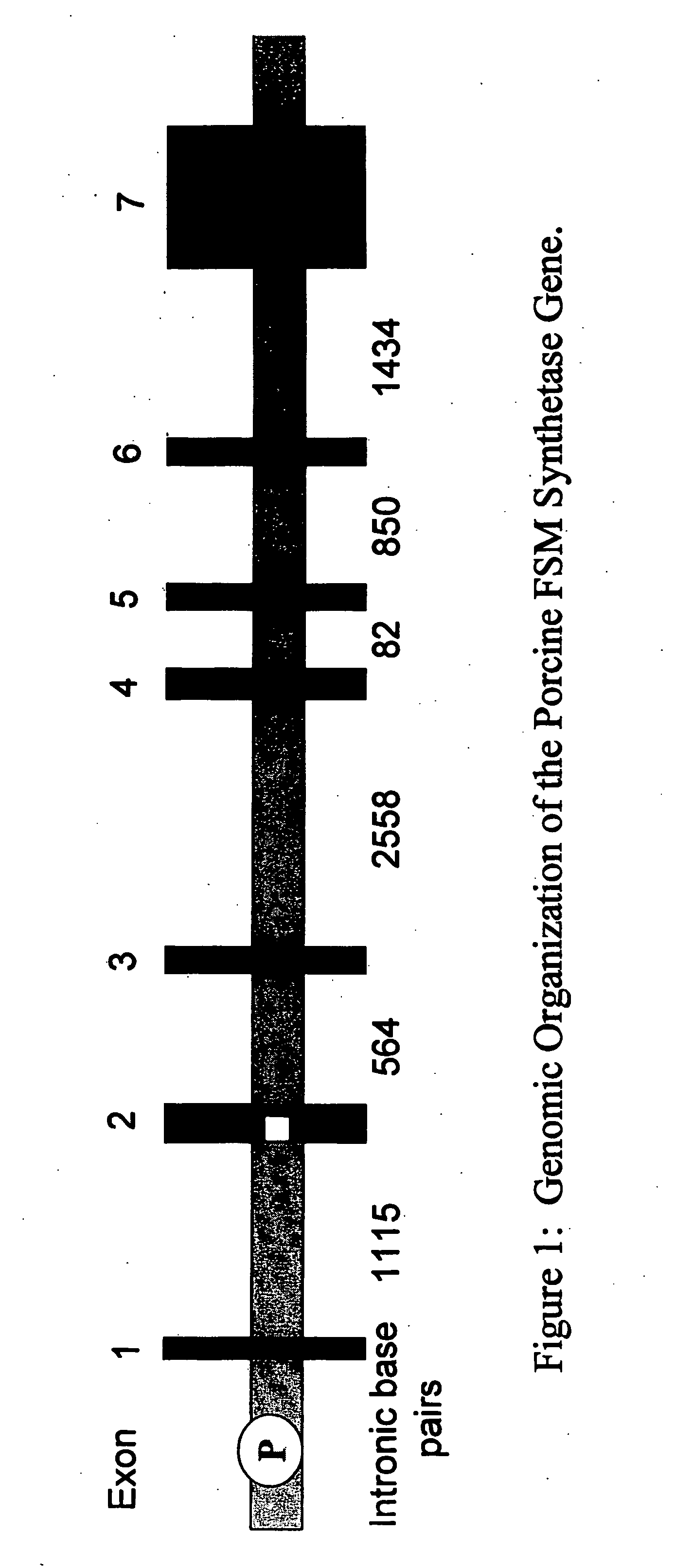
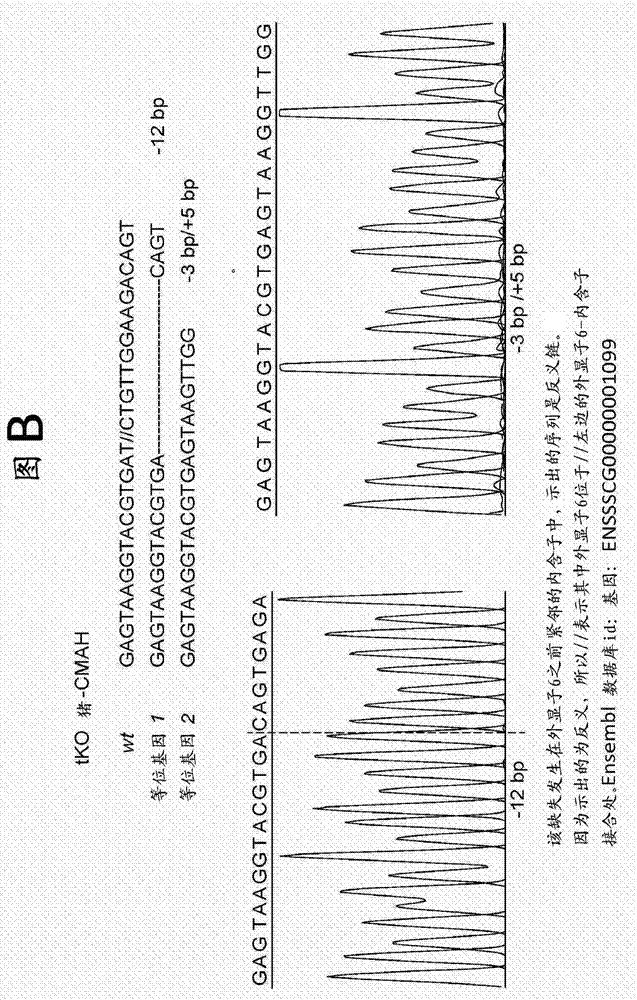
Porcine forssman synthetase protein, cDNA, genomic organization, and regulatory region
InactiveUS20060068479A1Low immunogenicityReduce expressionSugar derivativesTissue cultureN-AcetylgalactosaminyltransferasesGenomic DNA
The present invention provides porcine Forssman synthetase (FSM synthase) (Globoside α-N-acetylgalactosaminyltransferase) protein, cDNA, and genomic DNA sequence. Furthermore, the present invention includes porcine animals, tissue and organs as well as cells and cell lines derived from such animals, tissue and organs, which lack expression of functional FSM synthetase. Such animals, tissues, organs and cells can be used in research and in medical therapy, including in xenotransplantation. In addition, methods are provided to prepare organs, tissues, and cells lacking the porcine FSM synthetase gene for use in xenotransplantation.
Owner:UNIVERSITY OF PITTSBURGH
Multi-Transgenic Pig for Xenotransplantation
PendingUS20180249688A1Reduce and eliminate spontaneous aggregationDigestive systemDead animal preservationAnimal scienceMedicine
Owner:REVIVICOR INC
Triple transgenic pigs suitable for xenograft
PendingUS20170311579A1Improve rejectionIncrease durationNew breed animal cellsMammal material medical ingredientsBiotechnologyGene
The application provides methods of improving a rejection related symptom, reducing premature separation and methods of producing a compound of interest with an altered epitope profile are provided. Knockout pigs with a disrupted gene or genes, and porcine organs, tissues, and cells therefrom are provided.
Owner:INDIANA UNIV RES & TECH CORP
Therapeutic and diagnostic applications of prostatic acid phosphatase in prostate cancer
InactiveUS20060294615A1Good curative effectGood effectBiocideGenetic material ingredientsAndrogenMammal
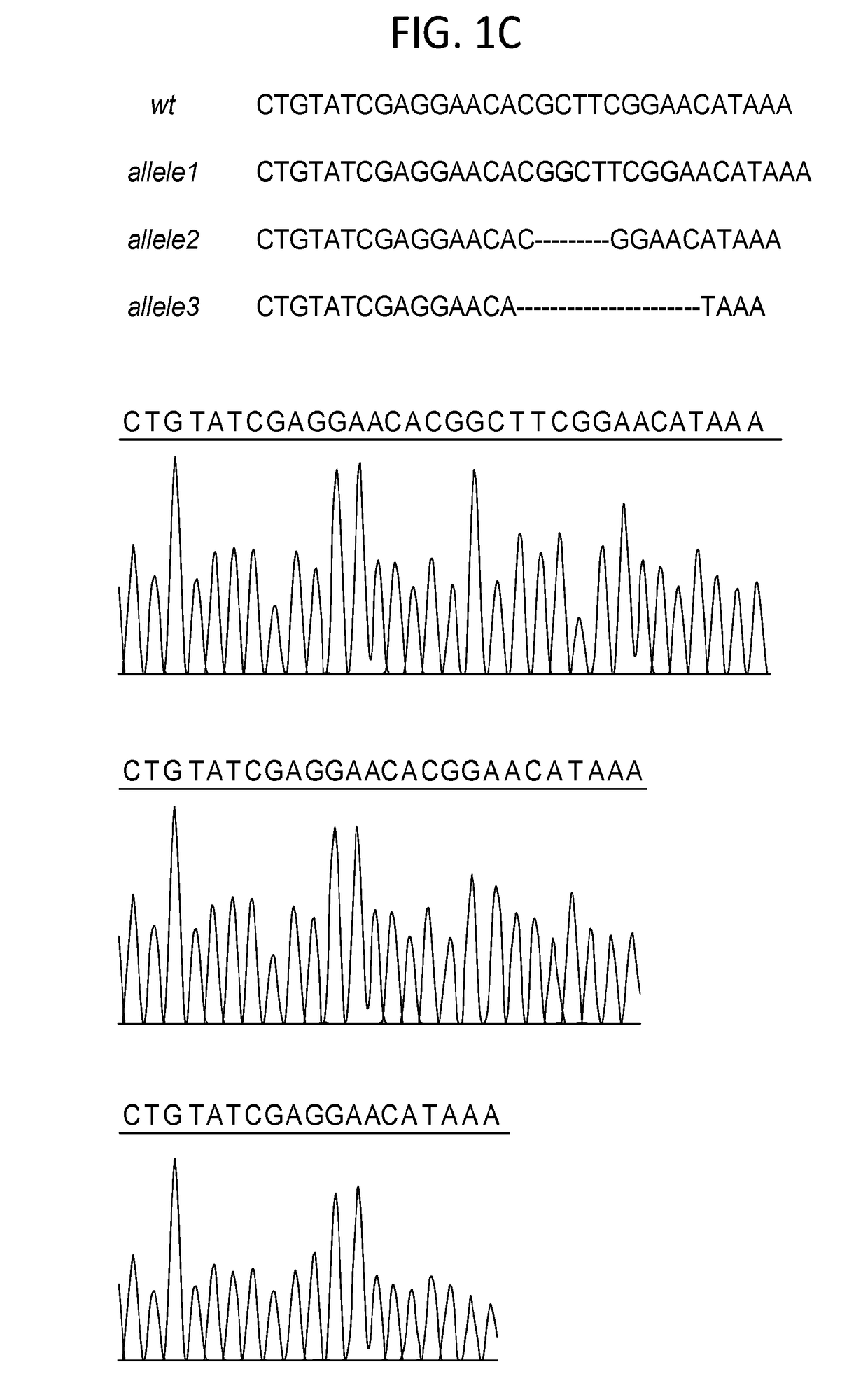
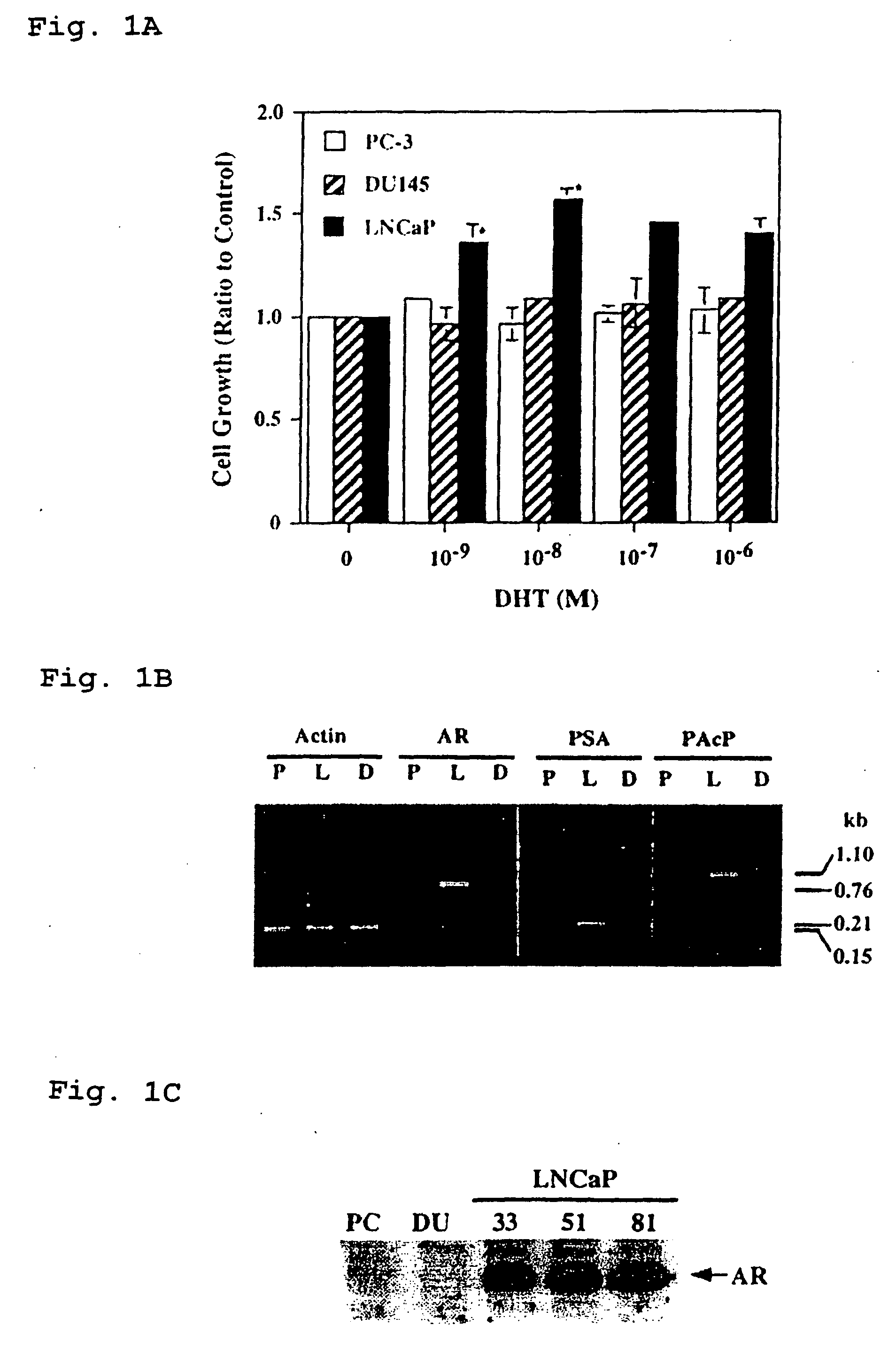
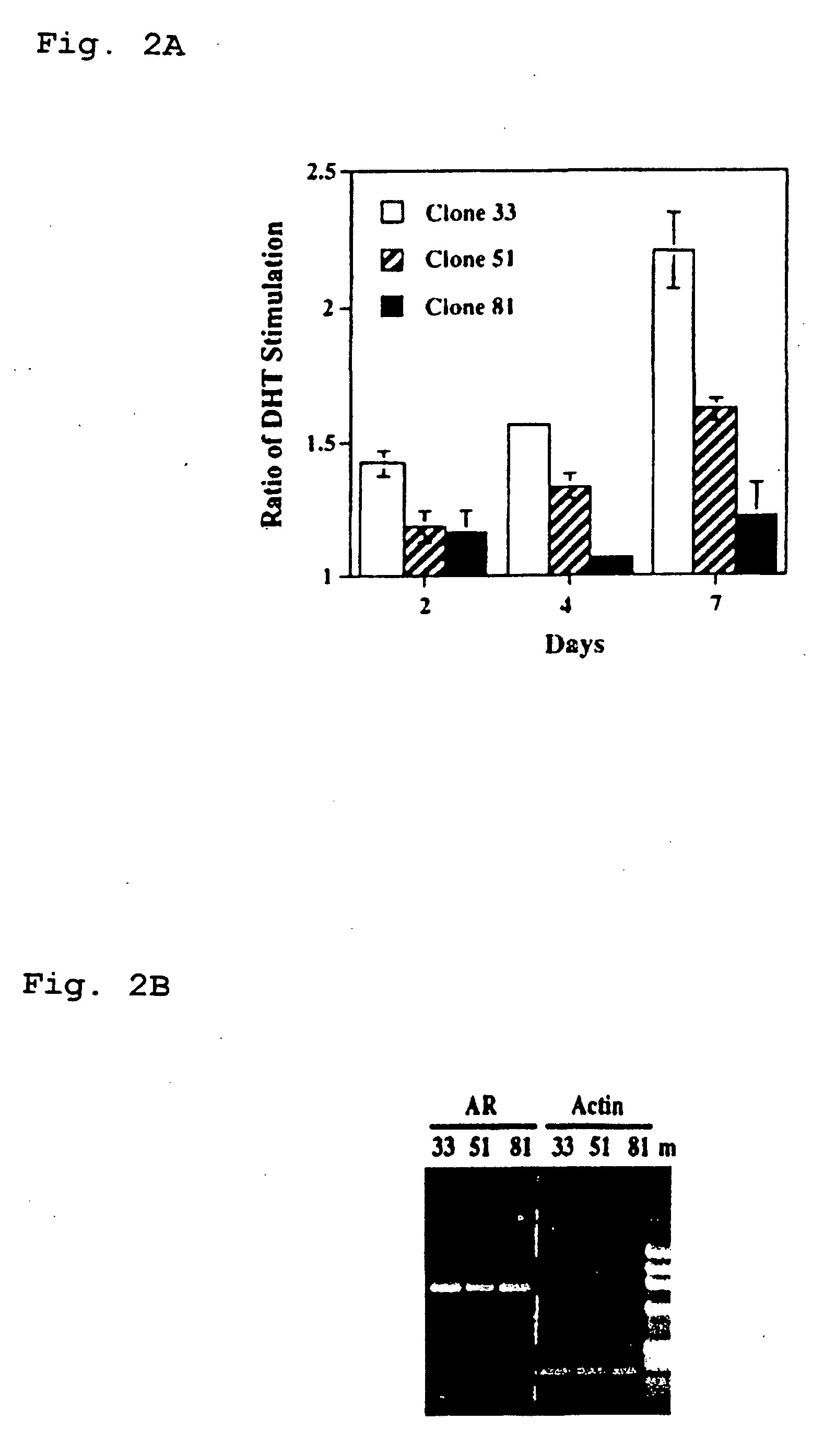
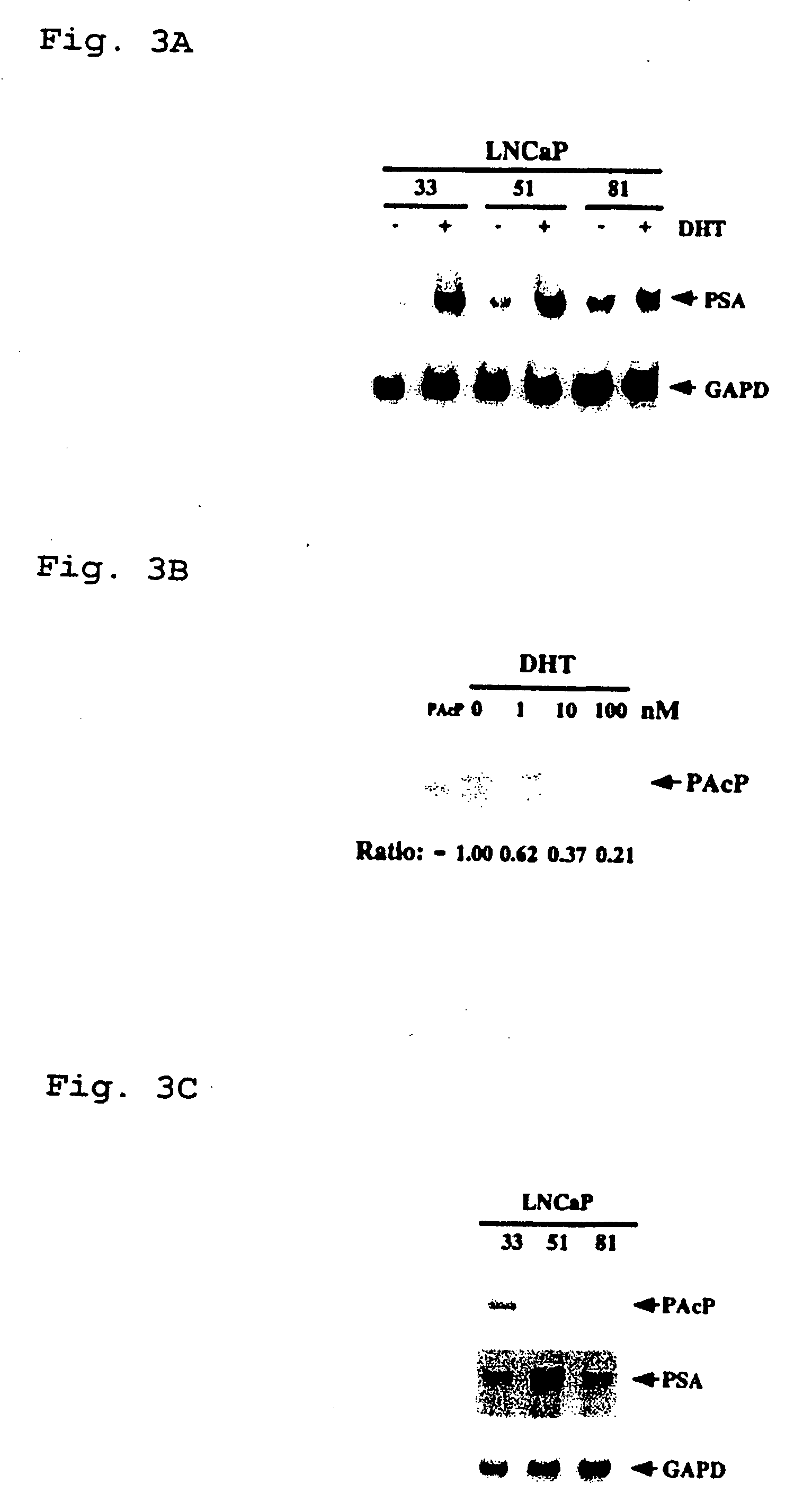
Presented is a therapeutic method to treat prostate carcinomas in mammals comprising the administration of cellular PAcP protein. Also presented is a method to diagnose androgen-insensitive prostate carcinomas by determining the expression level of cellular PAcP in the prostate carcinomas, a decrease in expression being indicative of androgen-insensitivity. A promoter region that is specifically expressed in prostate tissue is presented, as is a xenograft animal model that mimics human prostate carcinomas in the expression of cellular PAcP.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Modified Organs and Cells for Xenotransplantation
InactiveUS20070089178A1Maintain integrityMaintaining viabilityBiocideGenetic material ingredientsHuman bodyGenetically engineered
Owner:RBC BIOTECH
Xenograft soft tissue implants and methods of making
ActiveUS20130209572A1Reduce or even removeMammal material medical ingredientsProsthesisAntigenBiomedical engineering
The present application is directed to the field of implants comprising soft tissue for use in implantation in humans. The soft tissue implants of the present application are preferably obtained from xenograft sources. The present application provides a chemical process that sterilizes, removes antigens from and / or strengthens xenograft implants. The present techniques yield soft tissue implants having superior structural, mechanical, and / or biochemical integrity. The present application is also directed to processes for treating xenograft implants comprising soft tissues such as dermis, and to implants produced by such processes.
Owner:TUTOGEN MEDICAL INC
Pair of polypeptides specifically identifying porcine NFkappaBp65 gene, and coding gene and application thereof
The invention discloses a pair of polypeptides specifically identifying a porcine NFkappaBp65 gene, and a coding gene and an application thereof. The polypeptides comprise a polypeptide A and a polypeptide B; double-strand amino acids in the polypeptide A sequentially comprise amino acid residues having site numbers of 2-3, 36-37, 70-71, 104-105, 138-139, 172-173, 206-207, 240-241, 274-275, 308-309, 342-343, 376-377, 410-411, 444-445, 478-479, 512-513 and 546-547 in a sequence 3; and double-strand amino acids in the polypeptide B sequentially comprise amino acid residues having site numbers of 2-3, 36-37, 70-71, 104-105, 138-139, 172-173, 206-207, 240-241, 274-275, 308-309, 342-343, 376-377, 410-411, 444-445, 478-479, 512-513 and 546-547 in a sequence 5. The polypeptides can specifically identify the porcine NFkappaBp65 gene, and can be used for the knockout or reconstruction of the porcine NFkappaBp65 gene to obtain porcine disease resistance breeding materials, xenograft donors and human disease animal models.
Owner:青岛市畜牧兽医研究所
Porcine fgl2
InactiveUS20060078550A1Inhibitory activityPrevent thrombosisCompound screeningApoptosis detectionRegulator geneGene
The present invention relates to porcine fgl2 gene and protein and methods of modulating the gene and protein. The methods are useful in preventing thrombosis associated with the xenotransplantation of porcine organs or tissues.
Owner:LEVY GARY
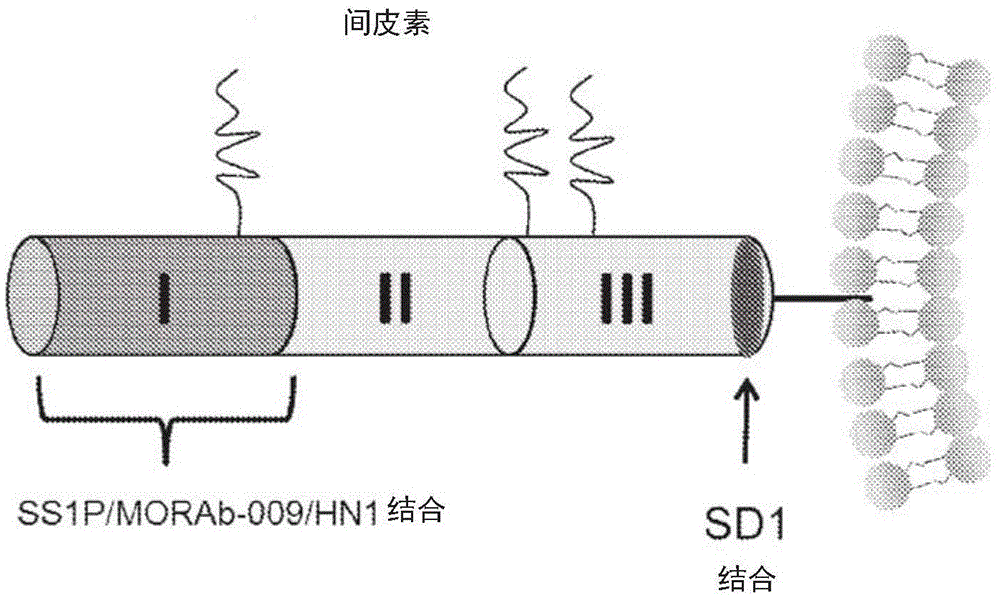
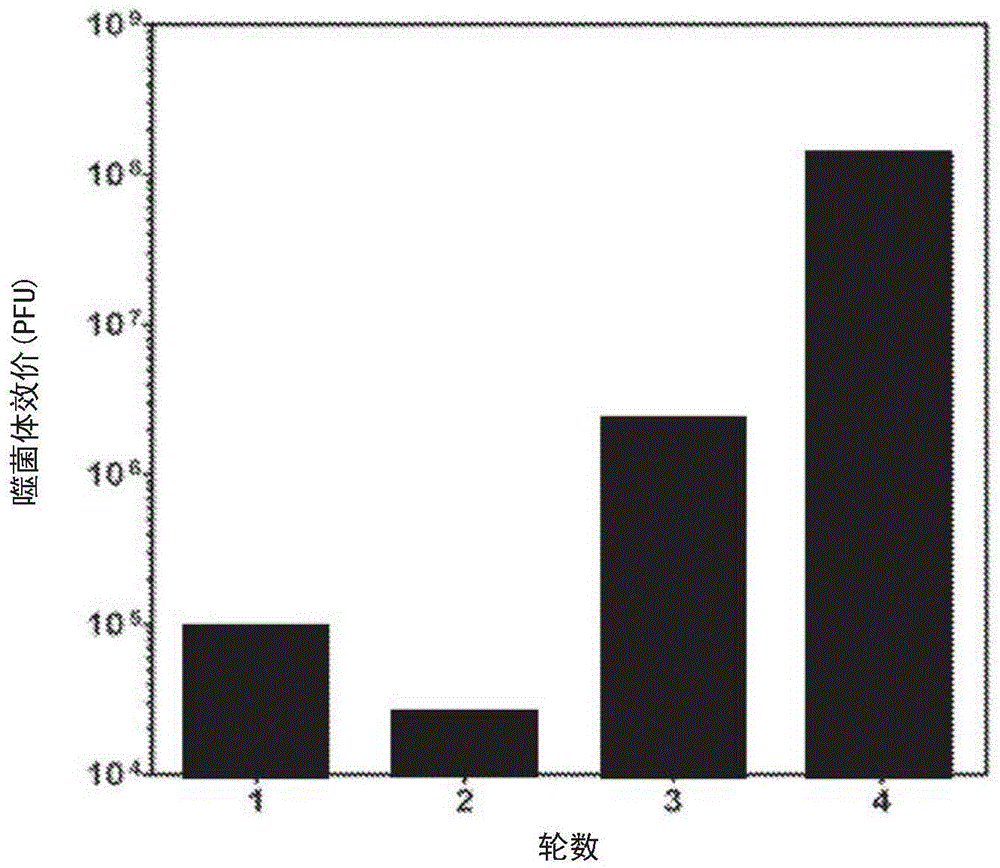
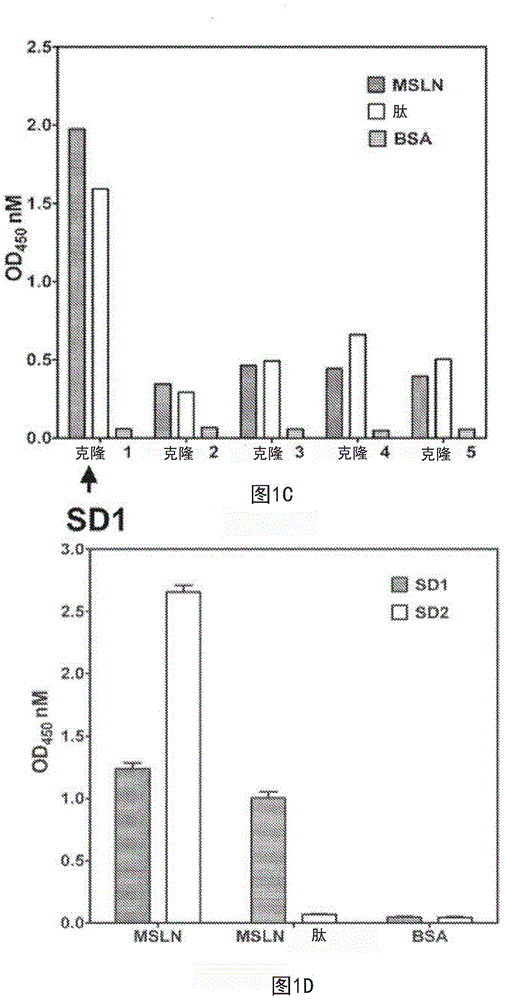
Mesothelin antibodies and methods for eliciting potent antitumor activity
ActiveCN104955845AAntibody mimetics/scaffoldsBiological material analysisSingle-domain antibodyTumor cells
Described herein is the use of phage display antibody engineering technology and synthetic peptide screening to identify SDl and SD2, human single-domain antibodies to mesothelin. SDl recognizes a conformational epitope at the C-terminal end (residues 539-588) of human mesothelin close to the cell surface. SD2 binds full-length mesothelin. To investigate SDl as a potential therapeutic agent, a recombinant human Fc (SDl-hFc) fusion protein was generated. The SDl-hFc protein exhibits strong complement-dependent cytotoxicity (CDC), in addition to antibody- dependent cellular cytotoxicity (ADCC), against mesothelin-expressing tumor cells. Furthermore, the SDl-hFc protein causes significant tumor growth inhibition of tumor xenografts in nude mice. SDl and SD2 are the first human single-domain antibodies targeting mesothelin-expressing tumors.
Owner:UNITED STATES OF AMERICA
Multi-transgenic pig for xenotransplantation
Owner:REVIVICOR INC
Adriamycin-indocyanine green bionic nanoparticles and application thereof
ActiveCN111000822AOrganic active ingredientsEnergy modified materialsDiseaseBreast cancer metastasis
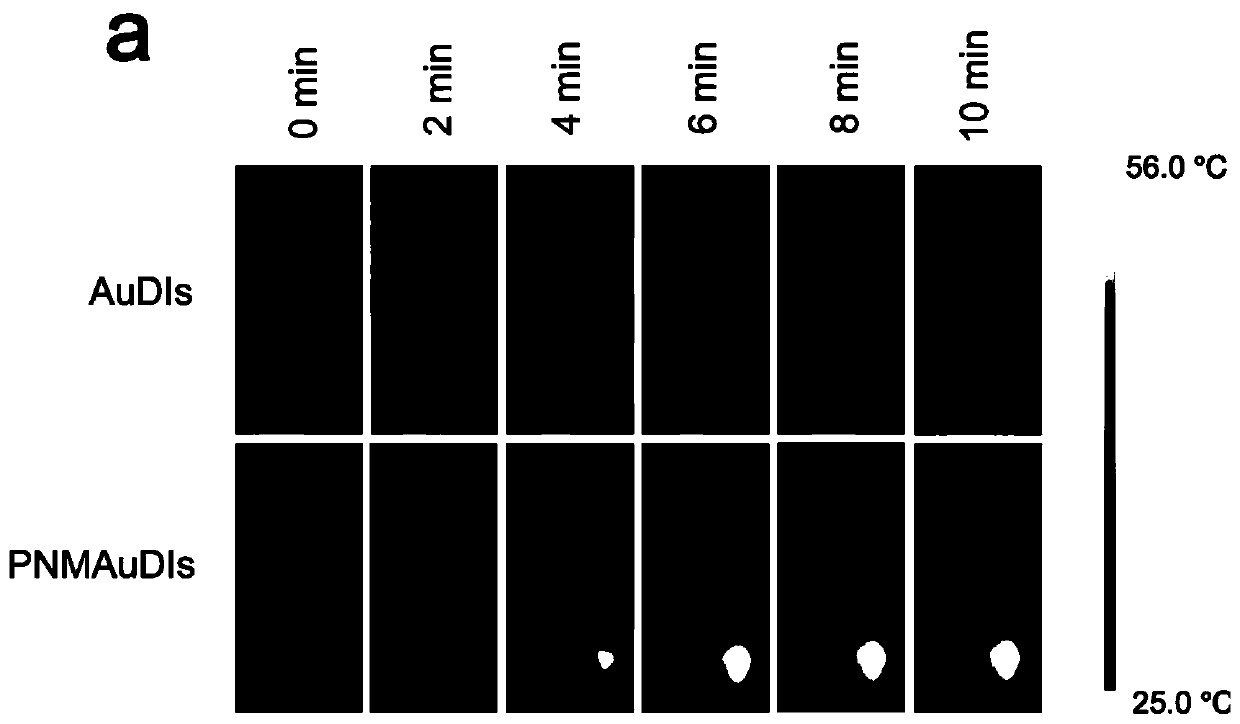
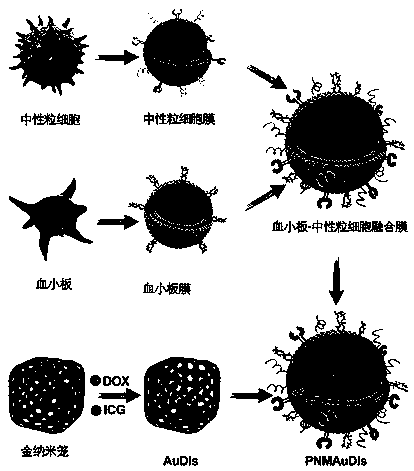
The invention relates to the technical field of medicines, and relates to adriamycin-indocyanine green bionic nanoparticles coated with platelet and neutrophil fusion membranes and an application of the adriamycin-indocyanine green bionic nanoparticles in preparation of medicines for treating tumor metastasis diseases. The bionic nanoparticle coated with the platelet and neutrophil hybrid membranecomprises adriamycin, indocyanine green, a nano carrier material, a platelet membrane and a neutrophil hybrid membrane, which is characterized by comprising the following components in percentage byweight: 8 to 10 percent of doxorubicin, 8 to 10 percent of indocyanine green, 30 to 40 percent of nano carrier material and the balance of platelet and neutrophile granulocyte hybrid membrane. The bionic nanoparticle has the capability of simultaneously capturing and removing circulating tumor cells and tumor-derived exosomes through a high-affinity membrane adhesion receptor, and effectively cutsoff the relationship between the exosomes and immune cells. The primary tumor can be completely ablated, and breast cancer metastasis can be efficiently inhibited in xenograft and in-situ breast tumor models.
Owner:SHENYANG PHARMA UNIVERSITY
Vivo assay for anti angiogenic compounds
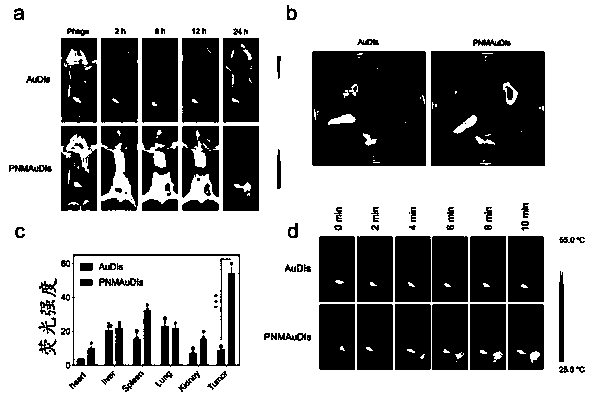
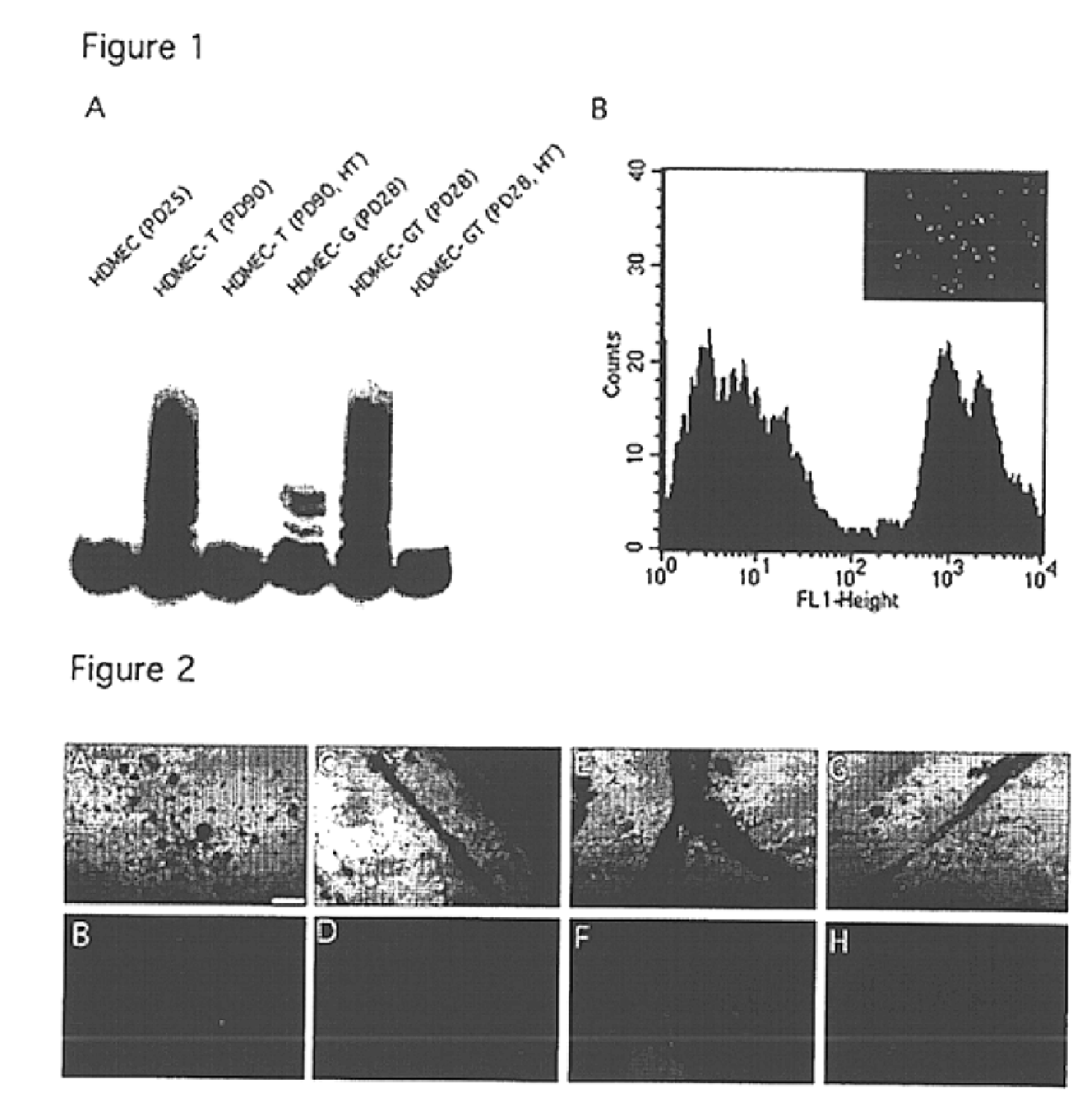
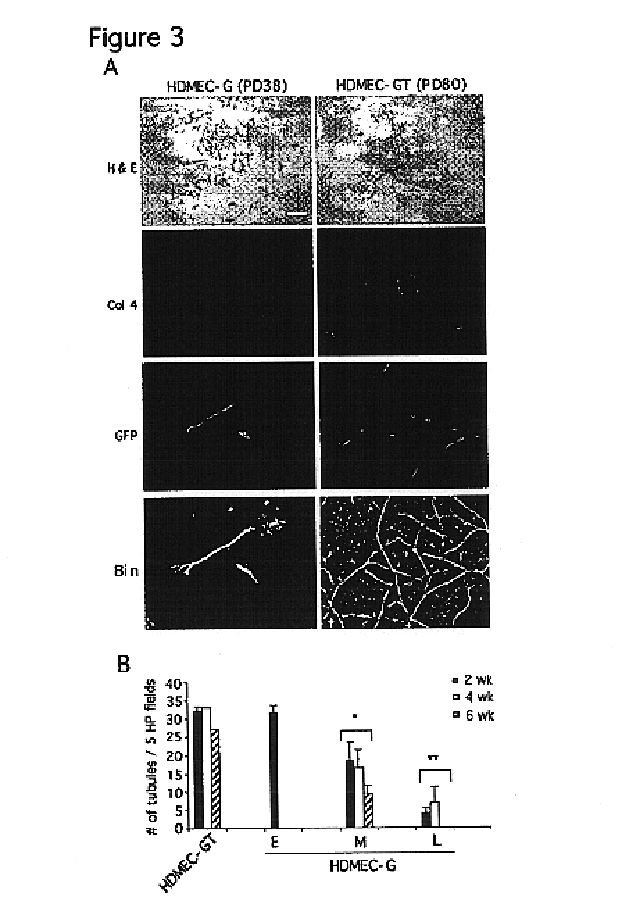
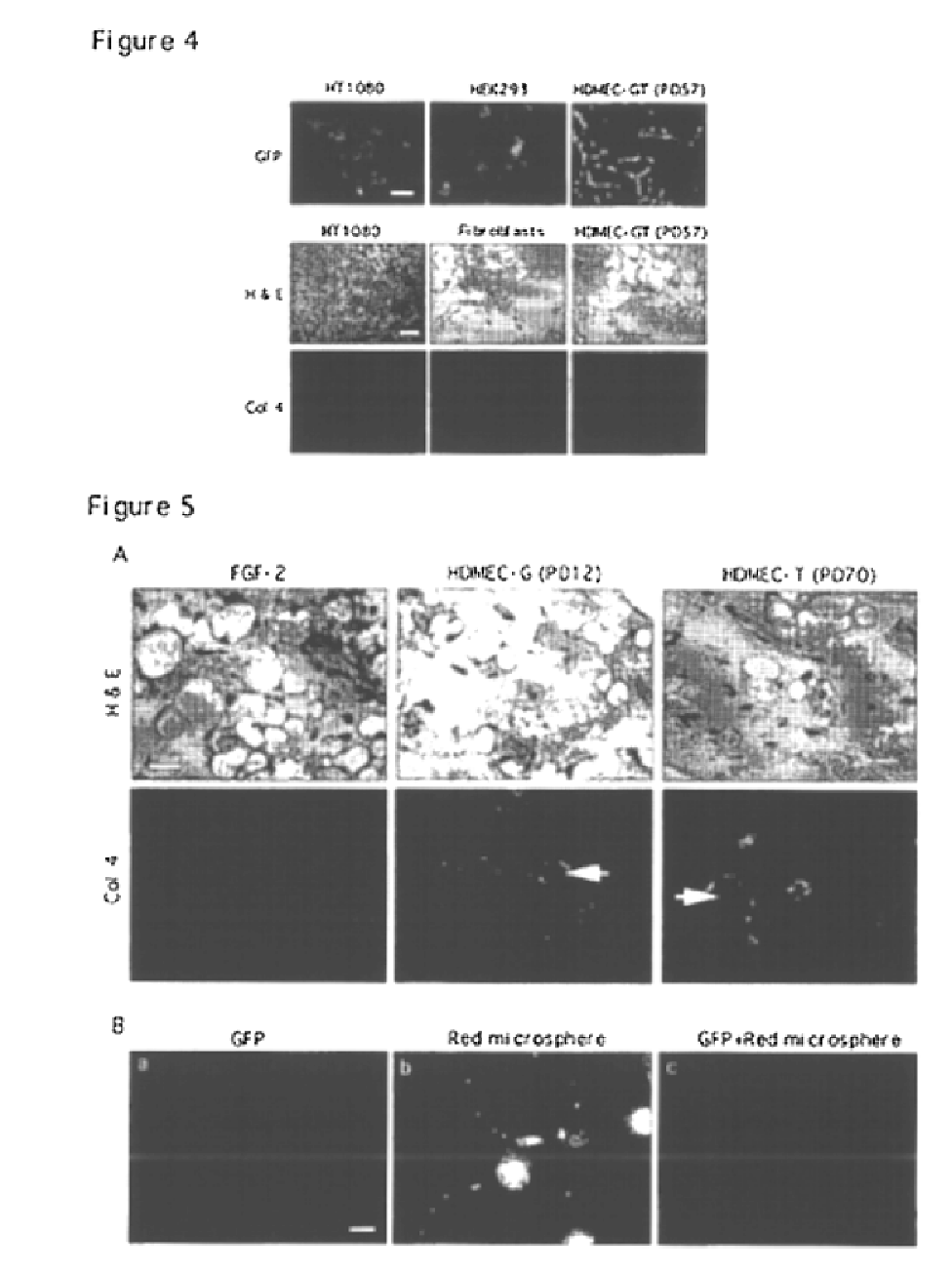
We report the use of telomerase-immortalized human microvascular endothelial cells in the formation of functional capillary blood vessels in vivo. Previously we showed the superior in vitro survival of human telomerase reverse transcriptase (hTERT)-transduced human endothelial cells. Here we show that retroviral-mediated transduction of hTERT in human dermal microvascular endothelial cells (HDMEC) results in cell lines that form microvascular structures when subcutaneously implanted in severe combined immunodeficiency (SCID) mice. The human origin of xenografted microvaculature was confirmed both by basement membrane immunoreactivity with anti-human type IV collagen staining and visualization of fluorescent vessels containing HDMEC that were co-transduced with hTERT and green fluorescent protein (eGFP). The lack of human vascular structures after implantation of HT1080 fibrosarcoma cells, 293 human embryonic kidney cells or human skin fibroblasts demonstrated the specificity of HDMEC at forming capillaries. Intravascular red fluorescent microspheres injected into the host circulation were found within green “telomerized” microvessels indicating functional murine-human vessel anastamoses. Whereas primary HDMEC-derived vessel density decreased steadily with time, telomerized HDMEC maintained durable vessels 6 weeks after xenografting. Modulation of implant vessel density by exposure to different angiogenic and angiostatic factors demonstrated the utility of this system for the study of human microvascular remodeling in vivo.
Owner:HERRON G SCOTT
Transgenic Ungulates Expressing CTLA4-IG and Uses Thereof
The present invention provides ungulates, including pigs, expressing CTLA4-Ig, as well as tissue, organs, cells and cell lines derived from such animals. Such animals, tissues, organs and cells can be used in research and medical therapy, including xenotransplanation. In addition, methods are provided to prepare organs, tissues and cells expressing the CTLA4-Ig for use in xenotransplantation, and nucleic acid constructs and vectors useful therein.
Owner:REVIVICOR INC
Pyrazolopyrimidinone kinase inhibitor
The present invention provides a novel pyrazolo[3,4-d]pyrimidin-4-one, specifically a derivative of 1-(pyridine-4-yl)-pyrazolo[3,4-d]pyrimidin-4-one. This compound is a kinase inhibitor that shows unexpected anti-proliferative activity against cells, including against tumor cells, and anti-tumor activity in xenograft tumor models. The compound or a suitable salt or prodrug thereof is useful for the treatment of individuals suffering from a cancer or another proliferative disorder or disease.
Owner:AGENNIX
ACE2 cell humanized mouse model as well as construction method and application thereof
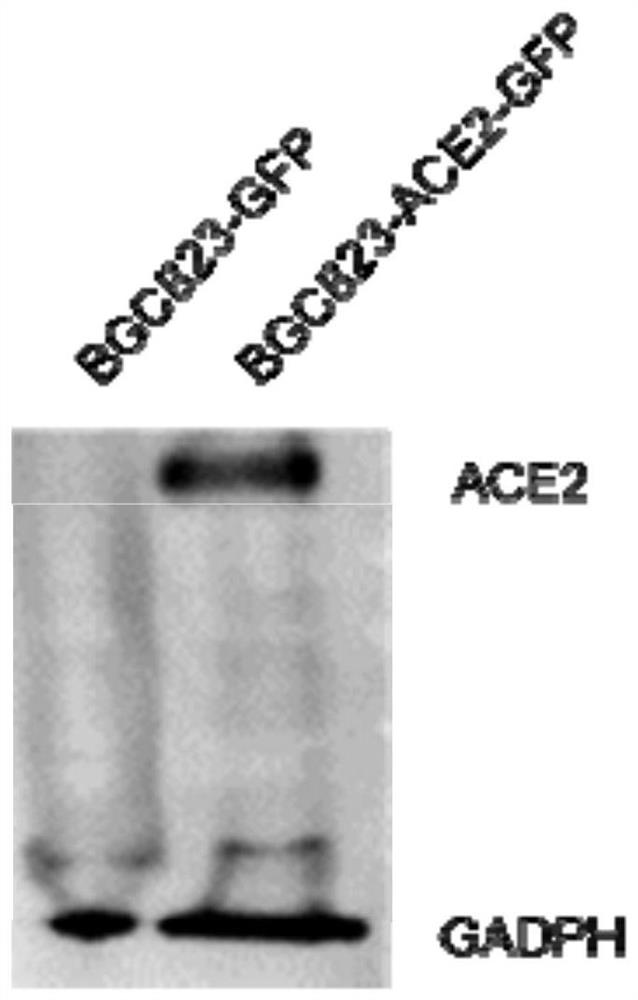
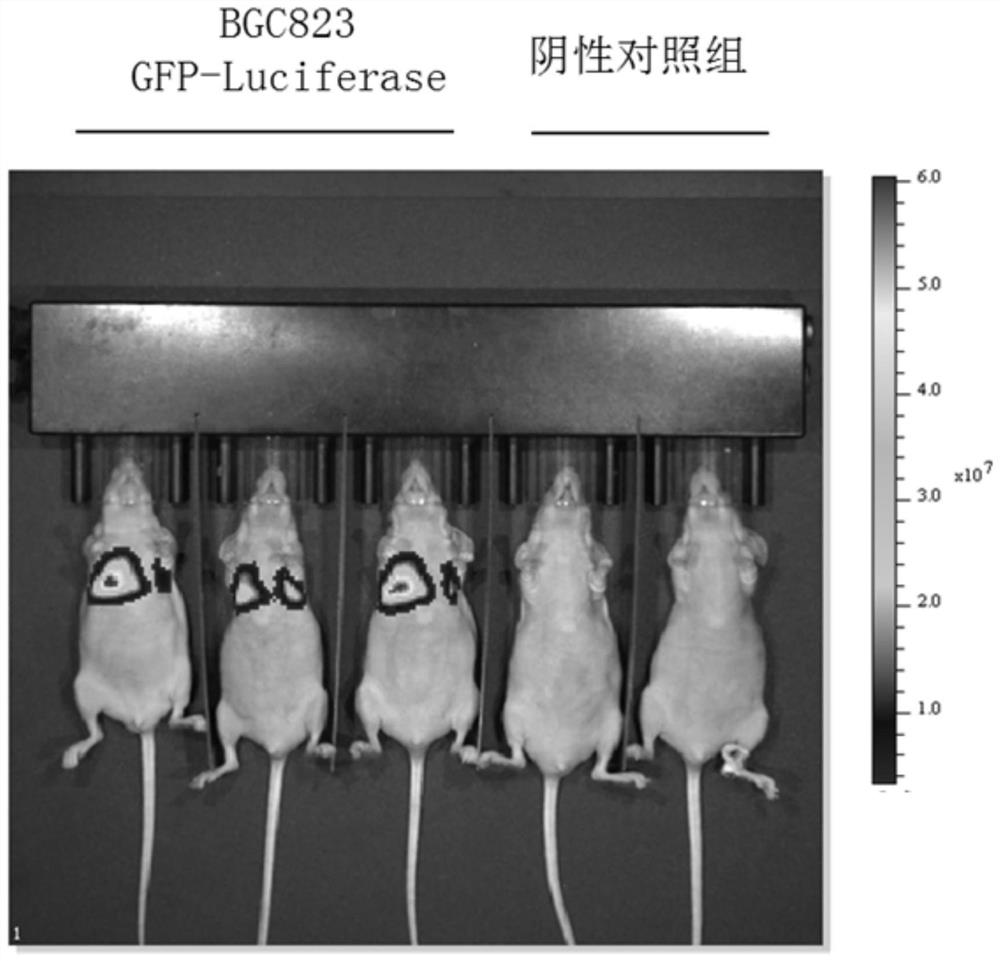
PendingCN111621523AHigh infection efficiencyAvoid xenograft rejectionFermentationVector-based foreign material introductionReceptorHuman cell
The invention provides an ACE2 cell humanized mouse model as well as a construction method and application thereof. The mouse model adopts an immunodeficient mouse as a parent and the body of the mouse contains humanized cells for overexpressing an ACE2 receptor. The mouse model can overexpress the ACE2 receptor in human cells in the body of the immunodeficient mouse, the infection efficiency of acoronavirus taking the ACE2 receptor as an infection medium is improved, meanwhile, xenograft rejection can be avoided, and background pollution is reduced. In addition, the mouse model can also be used for transplantation and immune reconstruction of human immune cells, and is of great significance for further research on the killing effect of the human immune system on the virus, screening of vaccines and evaluation of immune treatment means.
Owner:湖南昭泰生物医药有限公司
Triple transgenic pigs suitable for xenograft
PendingCN107106607AReduce the binding forceNew breed animal cellsMammal material medical ingredientsBiotechnologyEpitope
The application provides methods of improving a rejection related symptom, reducing premature separation and methods of producing a compound of interest with an altered epitope profile are provided. Knockout pigs with a disrupted gene or genes, and porcine organs, tissues, and cells therefrom are provided.
Owner:INDIANA UNIV RES & TECH CORP
Methods of preparation and composition of peptide constructs useful for treatment of autoimmune and transplant related host versus graft conditions
InactiveUS7256254B2Effectively eliminate set and subsetTreating or preventing inappropriate autoimmune responsePeptide/protein ingredientsPeptide sourcesCardiac myosinImmunologic disorders
Peptide constructs including a first peptide segment which includes an amino acid sequence associated with autoimmune disease, asthma, allergy or xeno- or allograft transplantation rejections bonded directly or via a linker or spacer to a second peptide which binds to T cells and which will redirect the immune response from a harmful Th1 response to a less harmful Th2 response, or which will bind to T cells to initiate, but not complete, an immune response causing the T cells to which the first peptide binds, to undergo anergy and apoptosis, are useful in treating autoimmune conditions. For instance, the peptide construct NGQEEKAGVVSTGLIGGGDSAFDVLSFTAEEKAGVYK (SEQ ID NO:14) wherein Th2 stimulating Peptide G (SEQ ID NO:15) is covalently linked, via spacer GGG, to cardiac myosin molecule My1 (SEQ ID NO:16), can be used for treatment or prevention of myocarditis.
Owner:CEL SCI CORP
Method for culturing 3-dimensional lung cancer organoid and method for preparing patient-derived xenograft animal model using same
PendingUS20210128752A1Maintenance characteristicCompounds screening/testingCell dissociation methodsAnticarcinogenOncology
The present invention relates to a method for culturing a 3-dimensional lung cancer organoid and a method for preparing a patient-derived xenograft animal model using the same. More specifically, the present invention relates to a method for culturing a 3-dimensional lung cancer organoid, a lung cancer organoid prepared by the method, a medium composition for culturing the lung cancer organoid, a method for preparing a xenograft animal model using the lung cancer organoid, a patient-derived lung cancer organoid xenograft animal model prepared by the method, and a method for analyzing therapeutic efficacy of an anticancer agent and a method for screening an anticancer agent, using the animal model.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION +1
Pre-clinical method for monitoring serial changes in circulating breast cancer cells in mice
InactiveUS20090117532A1Dead animal preservationMaterial analysisAbnormal tissue growthClinical study
The CellTracks® System provides a system to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. Pre-clinical studies of circulating tumor cells (CTC's) have been limited by the inability to repetitively monitor CTC's in animal models. The present invention provides a method to enumerate CTC's in blood samples obtained from living mice, using a protocol similar to an in vitro diagnostic system for quantifying CTC's in patients. Accordingly, this technology can be adapted for serial monitoring of CTC's in mouse xenograft tumor models of human breast cancer.
Owner:VERIDEX LCC
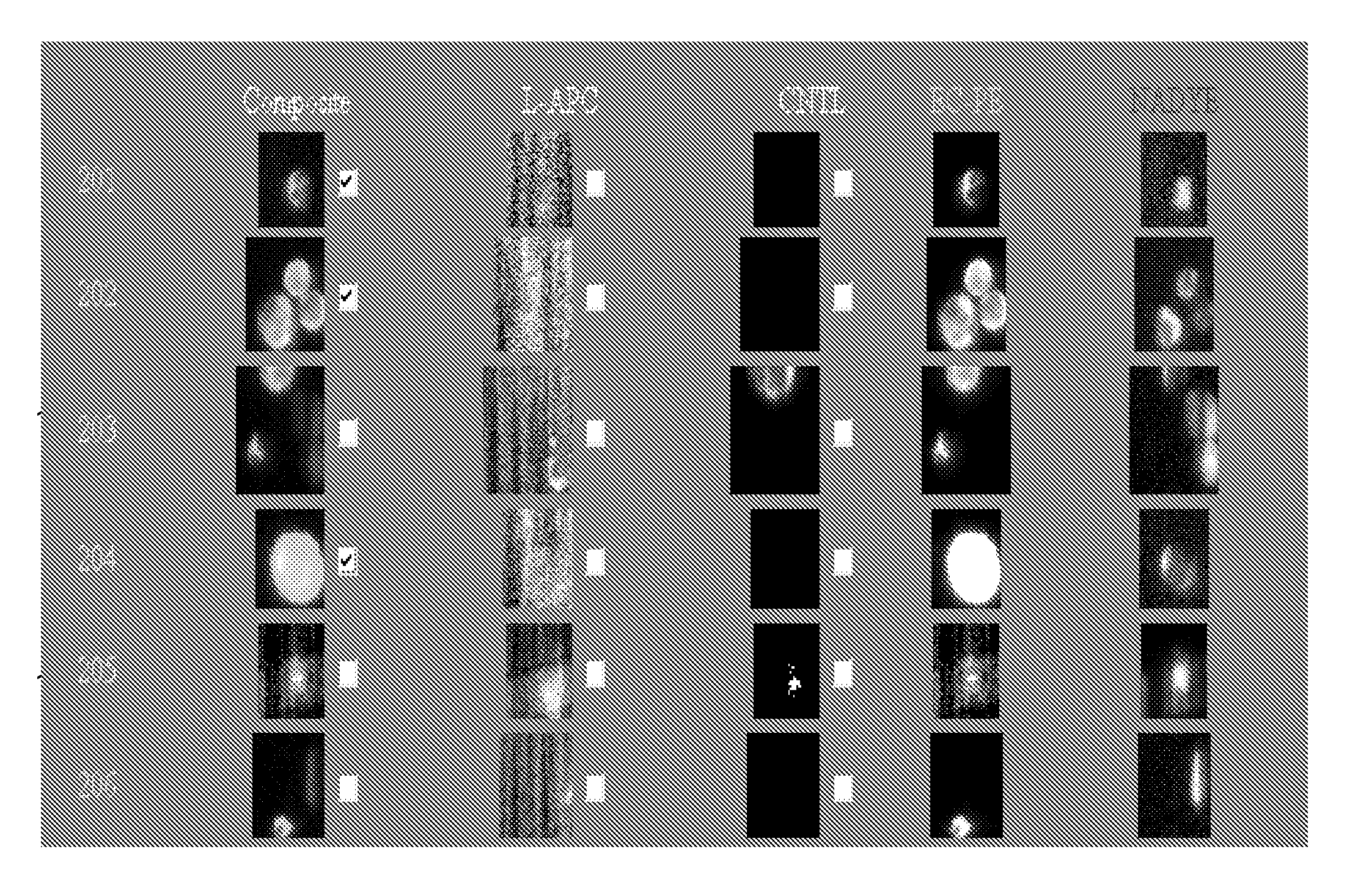
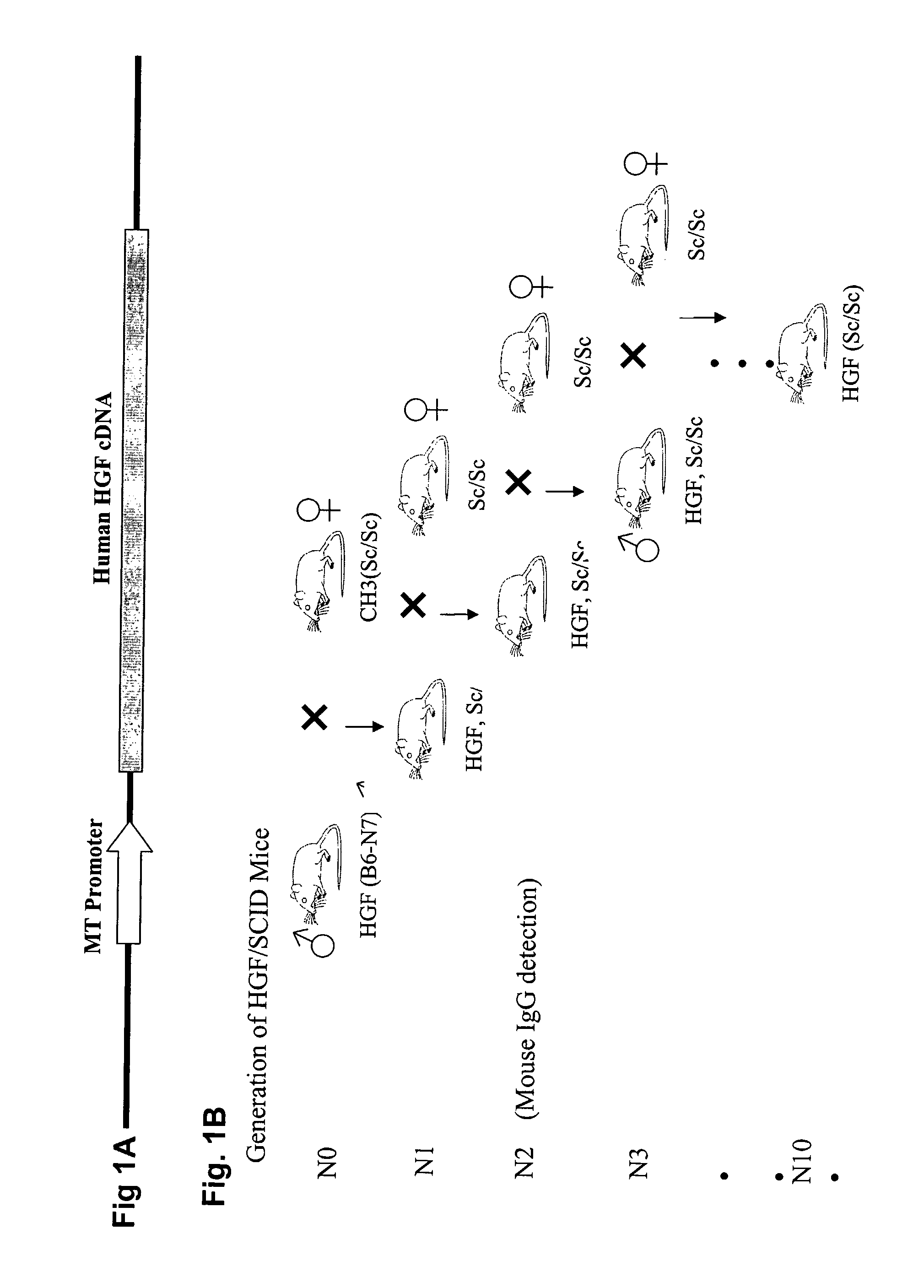
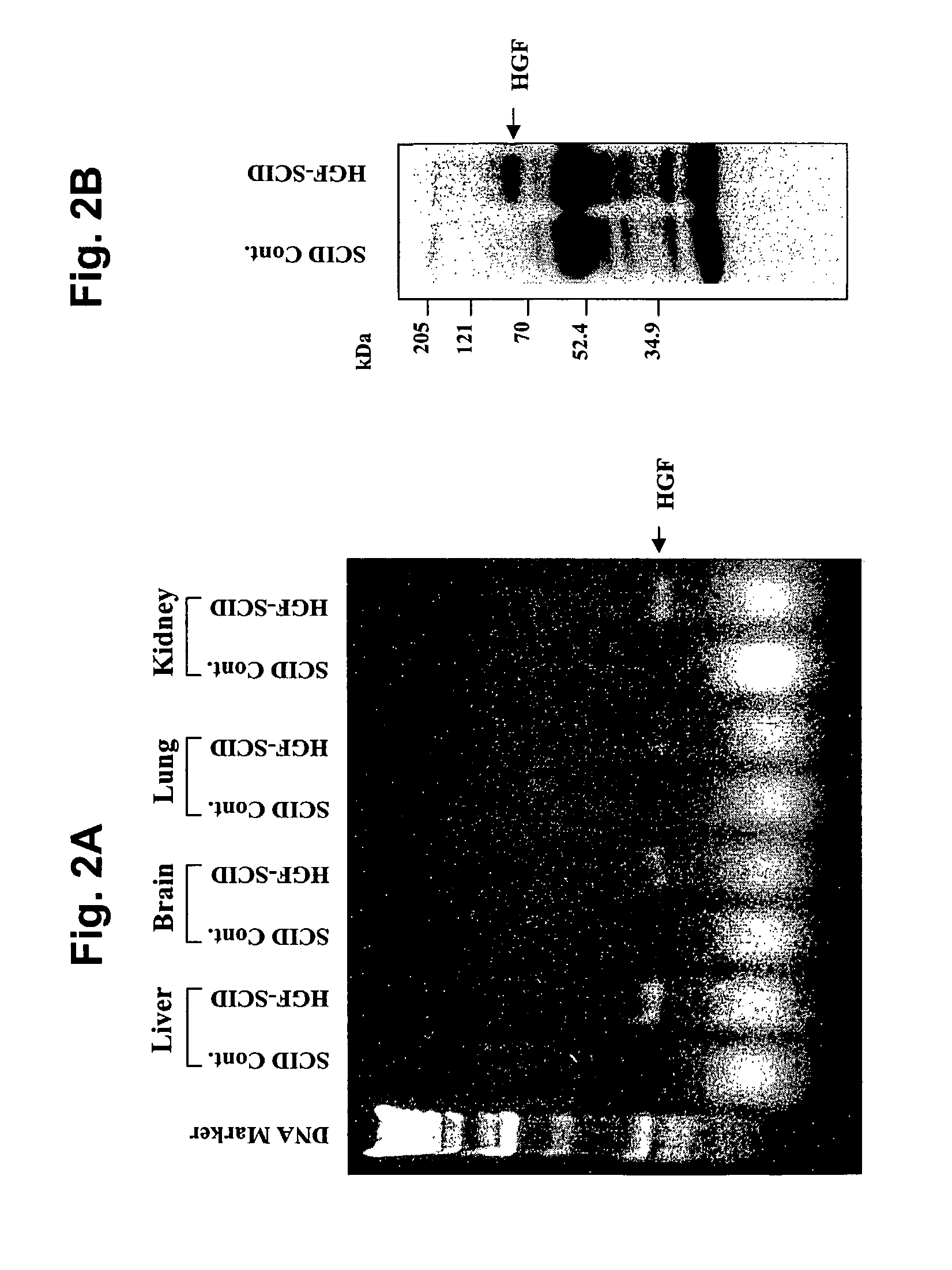
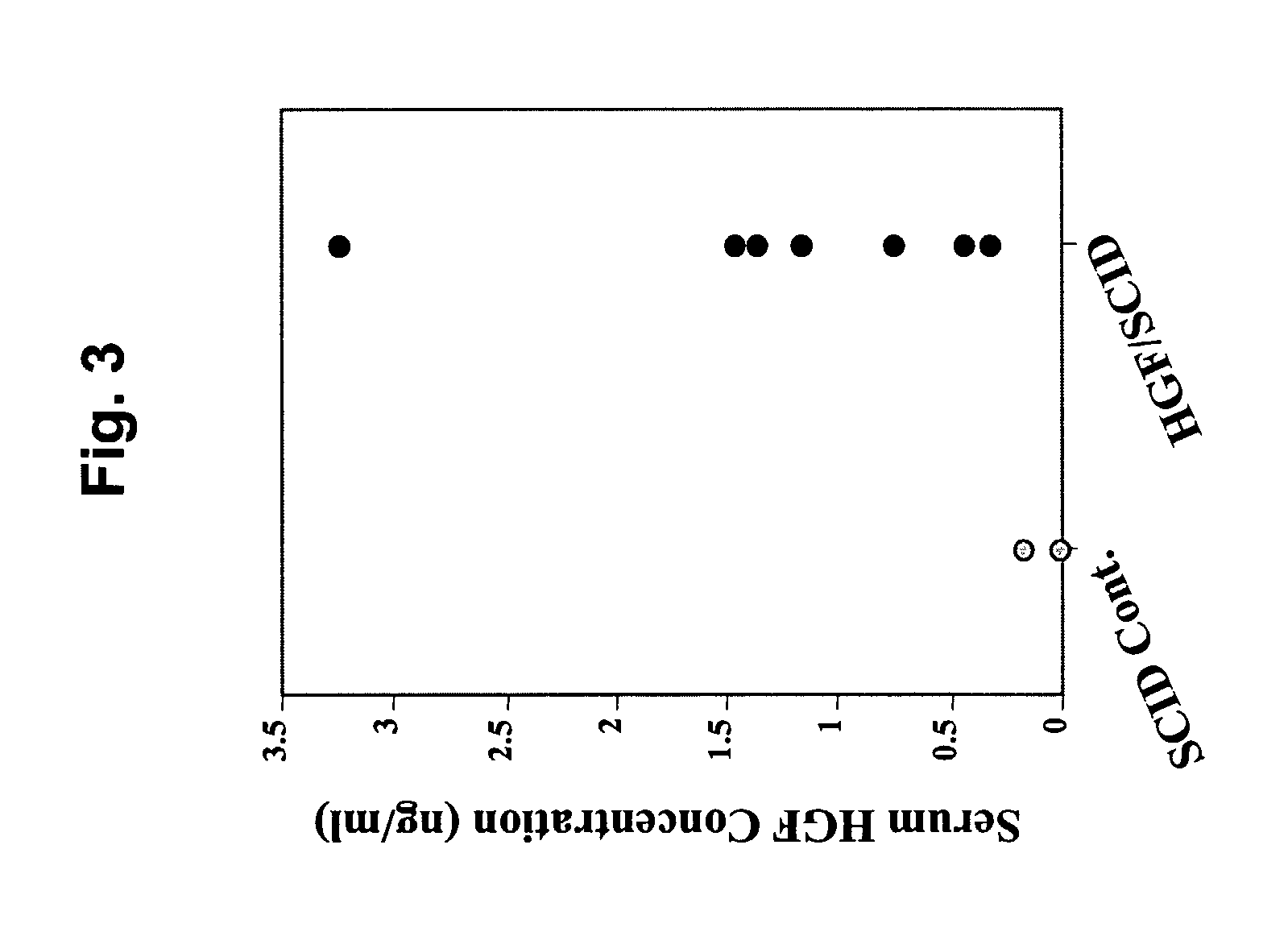
Immune-compromised transgenic mice expressing human hepatocyte growth factor (hHGF)
InactiveUS7968762B2Animal cellsHepatocyte-growth/scatter/tumor-cytotoxic factorImmune compromisedLymphatic Spread
A transgenic animal model for evaluating growth, survival and / or metastasis of xenotransplanted normal or tumor cells or tissue is disclosed, in which a human growth factor, hHGF stimulates growth in vivo of human cells or tissue. A strain of Tg mice on the C3H background that is immunocompromised as a result of a homozygous scid gene has been bred which express a nucleic acid encoding hHGF / SE The ectopically expressed hHGF / SF ligand significantly enhances growth of human tumor cell lines and explanted tumor cells or tissue that express the Met receptor for hHGF. Such animals also have an enlarged normal livers and greater than normal liver regenerative capacity. Any Met-expressing hHGF-dependent human cells, including hepatocytes and various stem cells can survive and grow in such animals.
Owner:VAN ANDEL RES INST
Spiral microchannel and use method thereof, and series-parallel installation structure
PendingCN109580323AImprove capture efficiencyEasy to handlePreparing sample for investigationLaboratory glasswaresRare cellCirculating cancer cell
The invention belongs to the field of rare cell or particle enrichment and screening, and relates to a spiral microchannel and a use method thereof, and a series-parallel installation structure. An inertial separation structure of a series-parallel spiral microchannel with reasonable cascading and application of multiple spiral microchannels are skillfully designed for the technical problem of thespiral microchannel in the field of sorting and enrichment of peripheral blood circulating tumor cells in a patient with a malignant tumor. Compared with the existing similar technology, the presentinvention not only has a good collection efficiency and processing flux of circulating tumor cells, but also maximizes the purity of circulating tumor cells and minimizes the loss of circulating tumorcells, and maintains the original phenotype of circulating tumor cells for sorting and enriching peripheral blood circulating tumor cells. The obtained cell suspension containing high-purity circulating tumor cells is particularly suitable for subsequent biomedical detection, genetic analysis, cell culture, xenograft tumor preparation, etc., and has extensive and far-reaching clinical practical application value.
Owner:中国人民解放军陆军特色医学中心
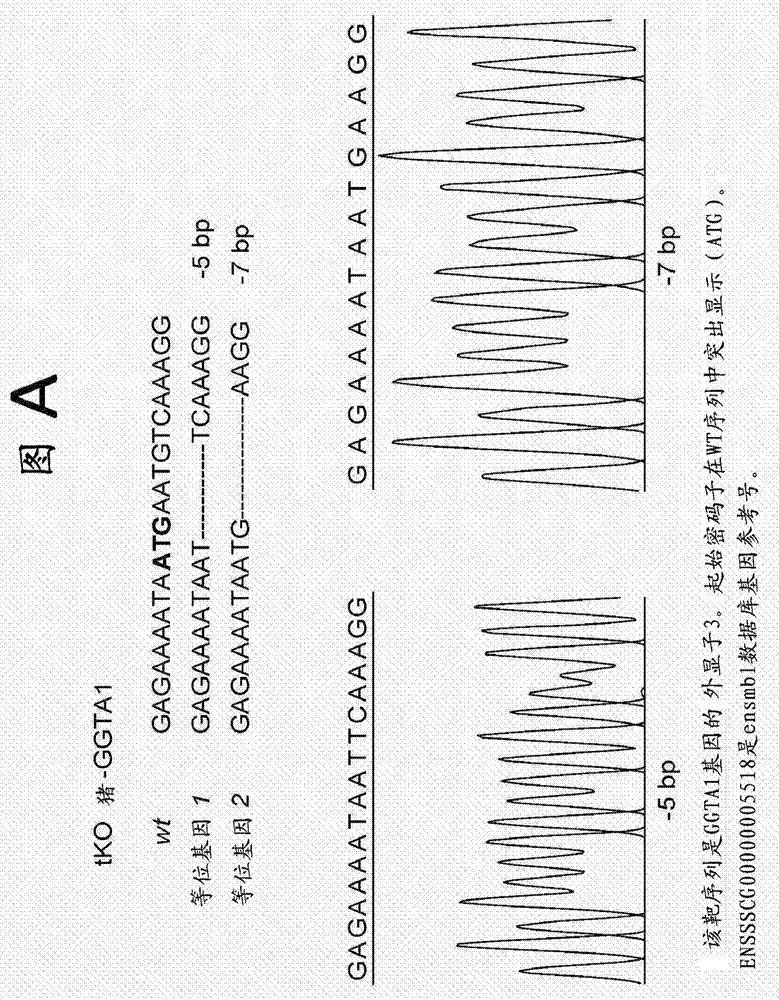
Method of preparing non-human tissues for xenotransplantation using α-galactosidase
This invention relates to novel α-galactosidases for the enzymatic removal of the immunodominant monosaccharides on blood products and tissues. Specifically this invention provides a novel family of α3 glycosidases, used for the enzymatic removal of type B antigens from blood group B and AB reactive blood products, and the GaIiIi antigen from non-human animal tissues, thereby converting these to non-immunogenic cells and tissues suitable for transplantation.
Owner:ORBIMED ADVISORS LLC
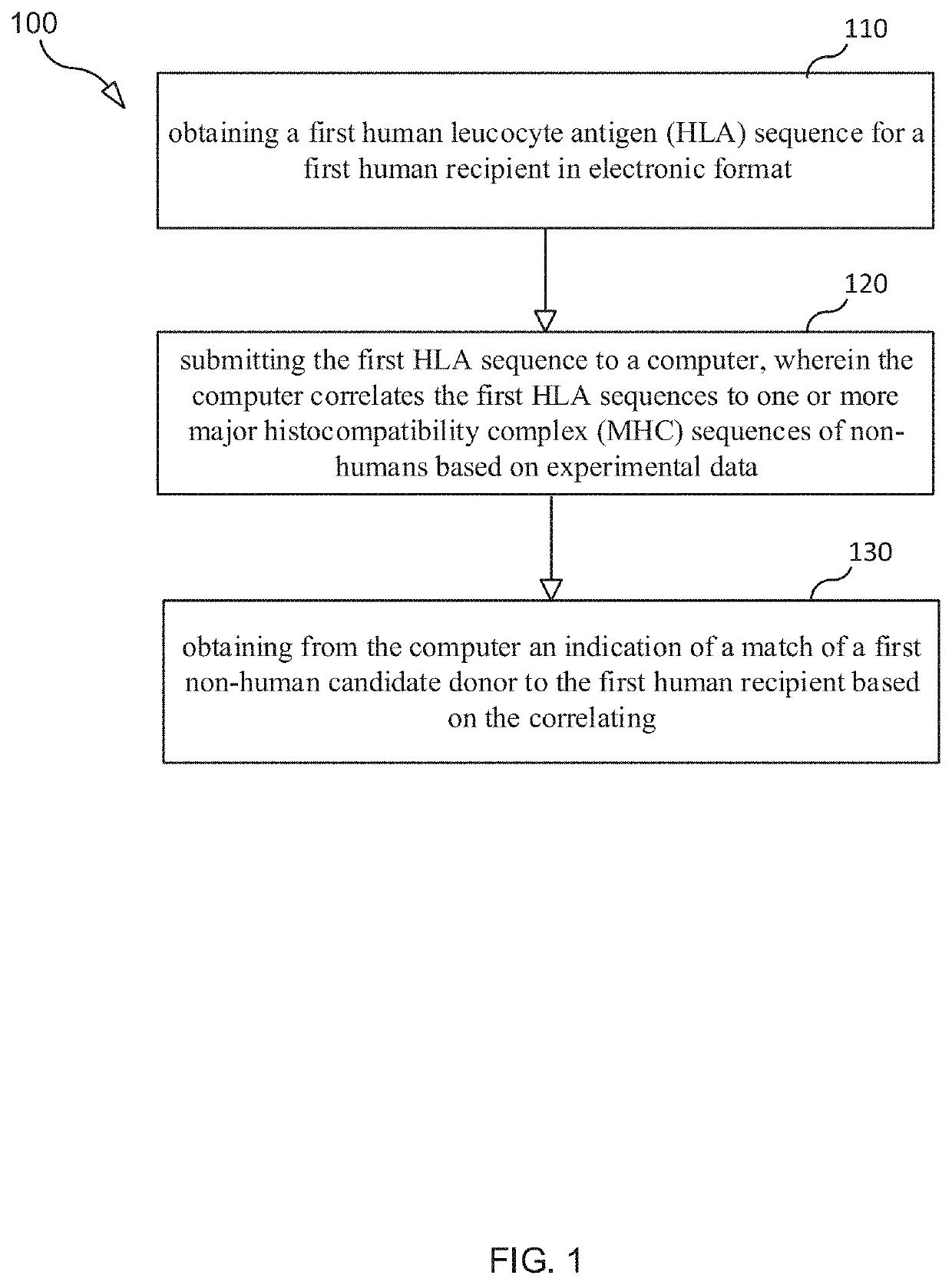
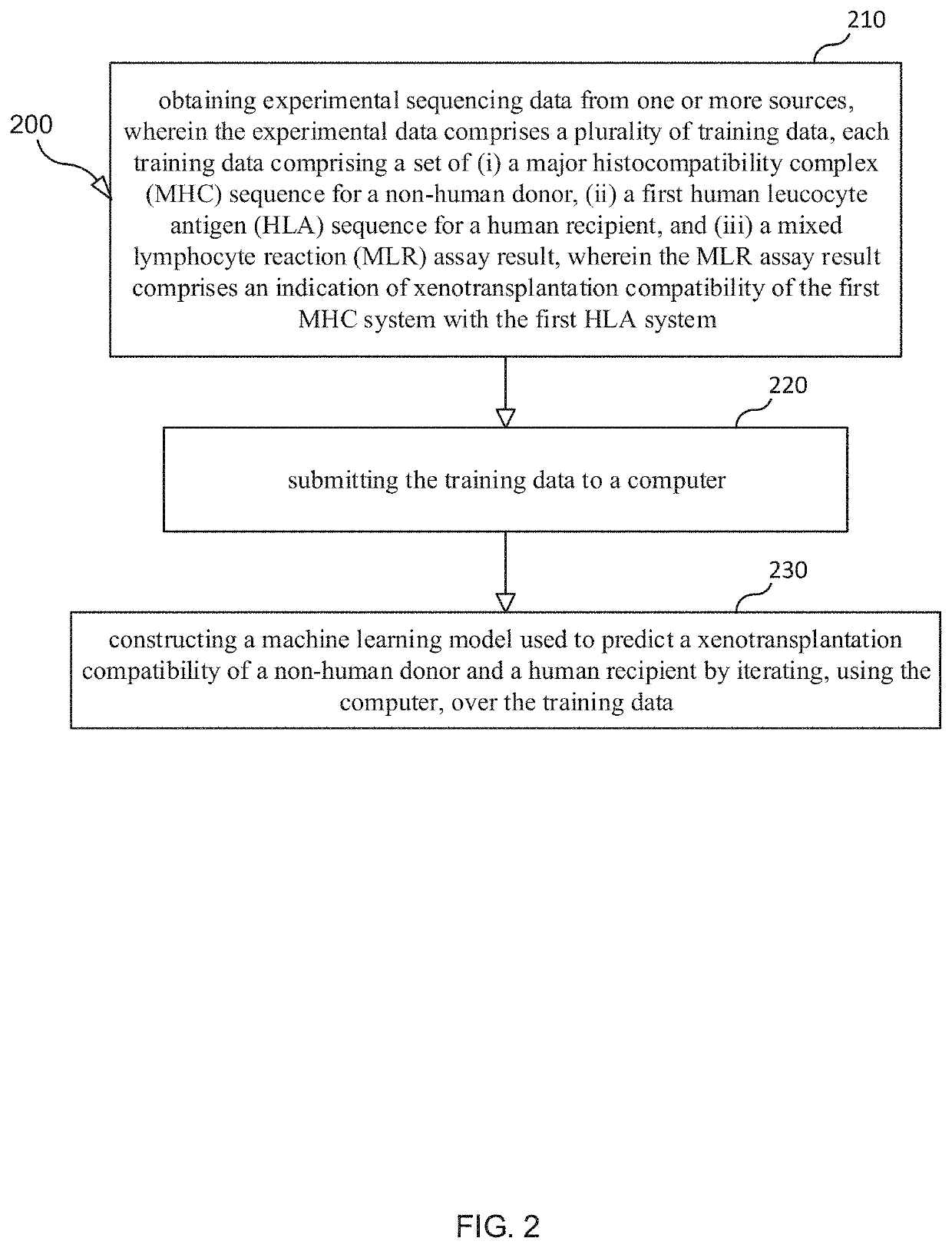
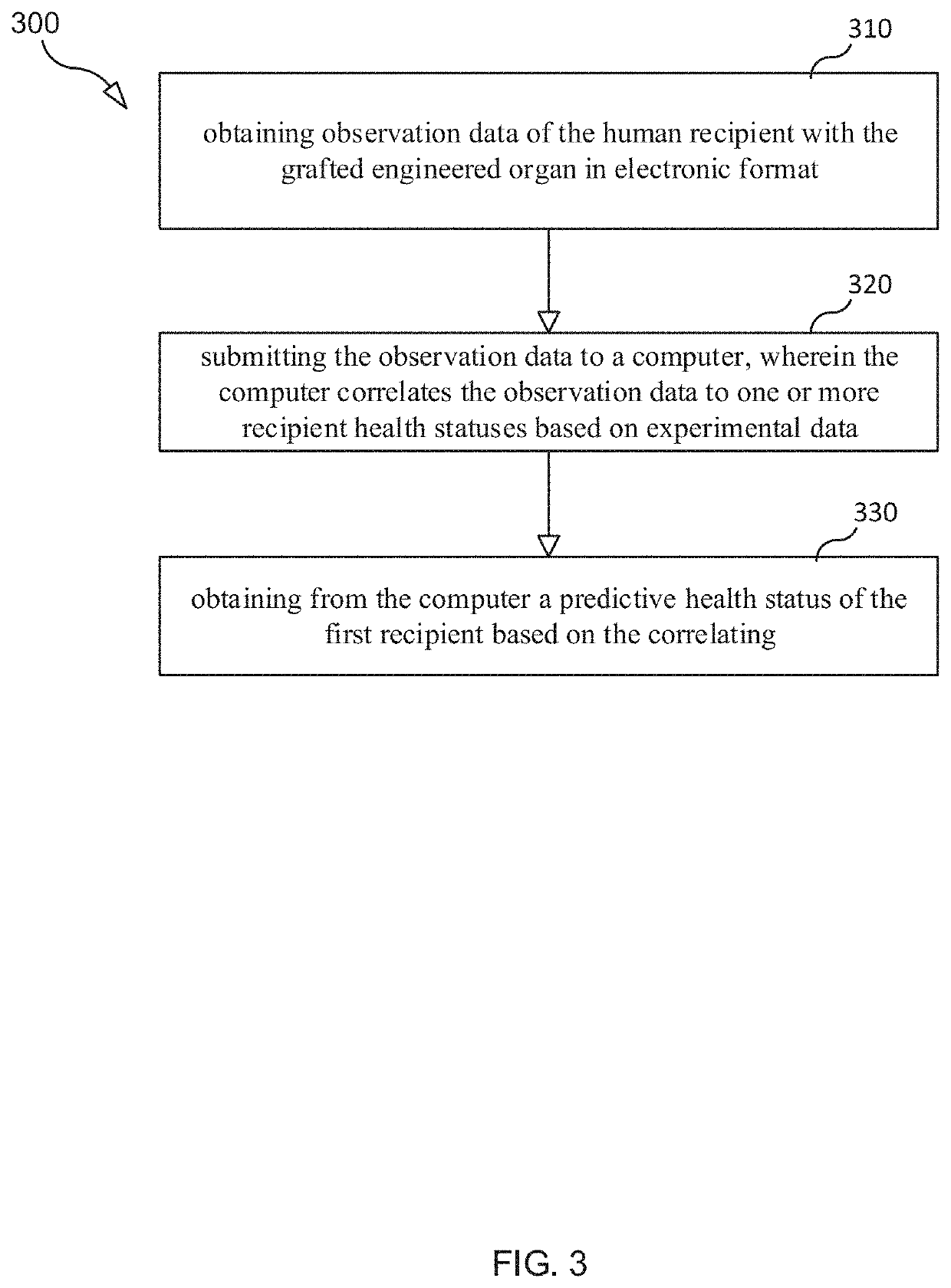
Selection and Monitoring Methods for Xenotransplantation
ActiveUS20210383892A1Efficiently exploreMaximize selectionMechanical/radiation/invasive therapiesDrug and medicationsData setResearch data
A method for predictive engineering of a sample derived from a genetically optimized non-human donor suitable for xenotransplantation into a human having improved quality or performance is provided. The method includes constructing a training data set from a series of libraries, wherein at least one library in the series of libraries comprises genomic, proteomic, and research data specific to non-humans. The method includes developing a predictive machine learning model based on the constructed training data set. The method includes utilizing the predictive machine learning model to obtain a predicted quality or performance of a plurality of sequences for a candidate sample from the non-human donor specific to a human patient or patient population. The method includes selecting a subset of sequences for evaluation from the plurality of sequences based on the predicted quality or performance. The method includes designing candidate samples derived from the non-human donor using the selected subset of sequences. The method includes measuring a respective in silico performance of each designed candidate sample. The method includes selecting a designed candidate sample for manufacture based on the respective in silico performance of each designed candidate sample.
Owner:XENOTHERAPEUTICS INC
Antigenic fusion protein carrying Galalpha 1,3Gal epitopes
InactiveUS20050255099A1Easy and cheap to produceSimple designOrganic active ingredientsPeptide/protein ingredientsEpitopeHeterografts
Owner:RECOPHARMA AB
Myoblast treatment of diseased or weakened organs
InactiveUS20070009499A1Increase blood flowImprove bindingBiocidePeptide/protein ingredientsHeterograftsWeakness
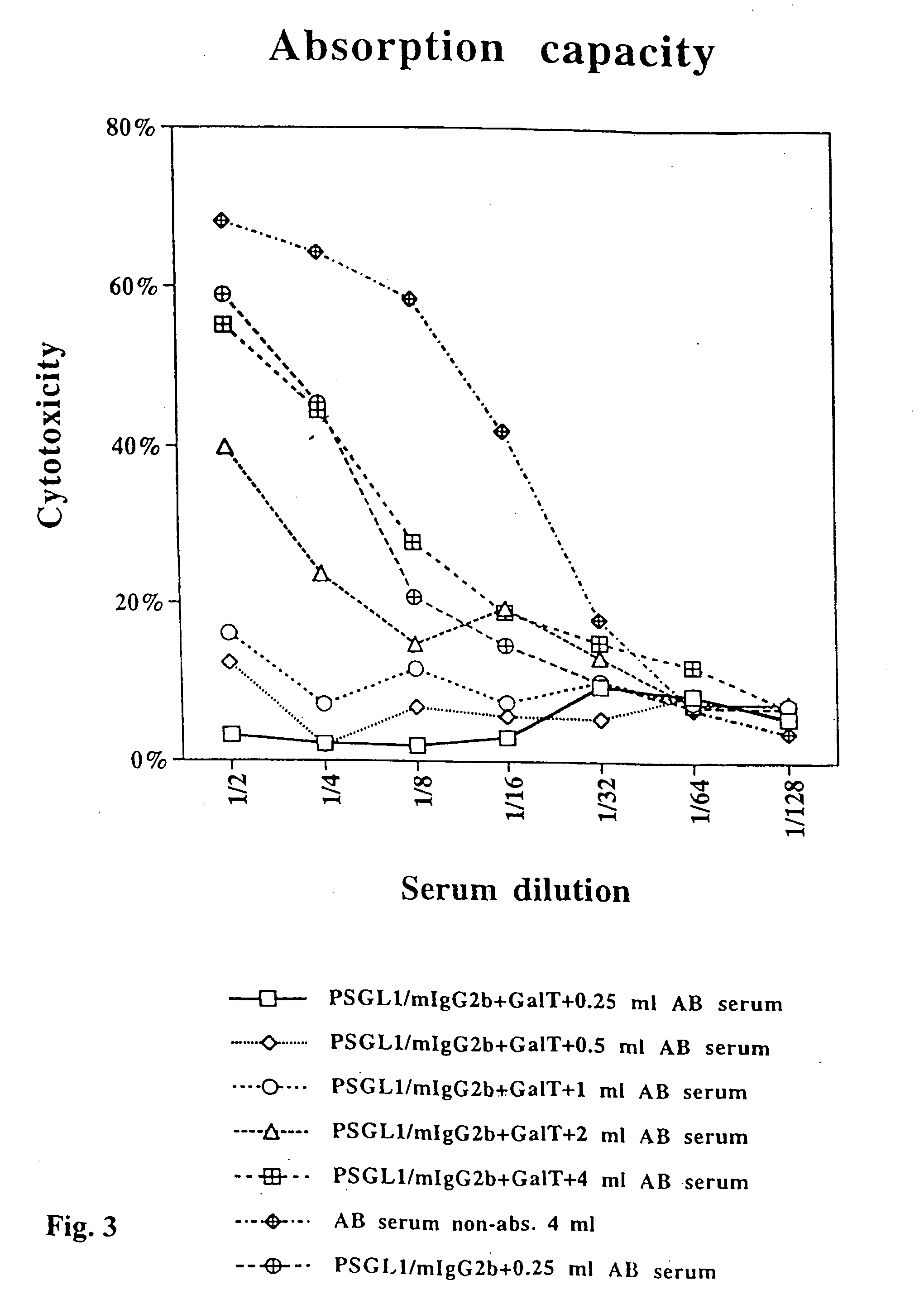
Bioengineering the regenerative heart or other body organ in need of greater muscle mass or improved blood perfusion provides a novel treatment for organ weakness or failure. In the case of cardiac failure treatment, on May 14, 2002, a 55-year-old man suffering ischemic myocardial infarction received 25 injections carrying 465 million cGMP-produced pure myoblasts into his myocardium after coronary artery bypass grafting. Three myogenesis mechanisms were elucidated with 17 human / porcine xenografts using cyclosporine as immunosuppressant. Some myoblasts developed to become cardiomyocytes. Others transferred their nuclei into host cardiomyocytes through natural cell fusion. As yet others formed skeletal myofibers with satellite cells. De novo production of contractile filaments augmented heart contractility. Human myoblasts transduced with VEGF165 gene produced six times more capillaries in porcine myocardium than placebo. Xenograft rejection was not observed for up to 20 weeks despite cyclosporine discontinuation at 6 weeks.
Owner:LAW PETER
Materials and methods for nerve repair with animal-sourced grafts
ActiveUS20200155156A1Overcomes shortcomingPromote repairSurgeryTissue regenerationHeterograftsIntercostal nerves
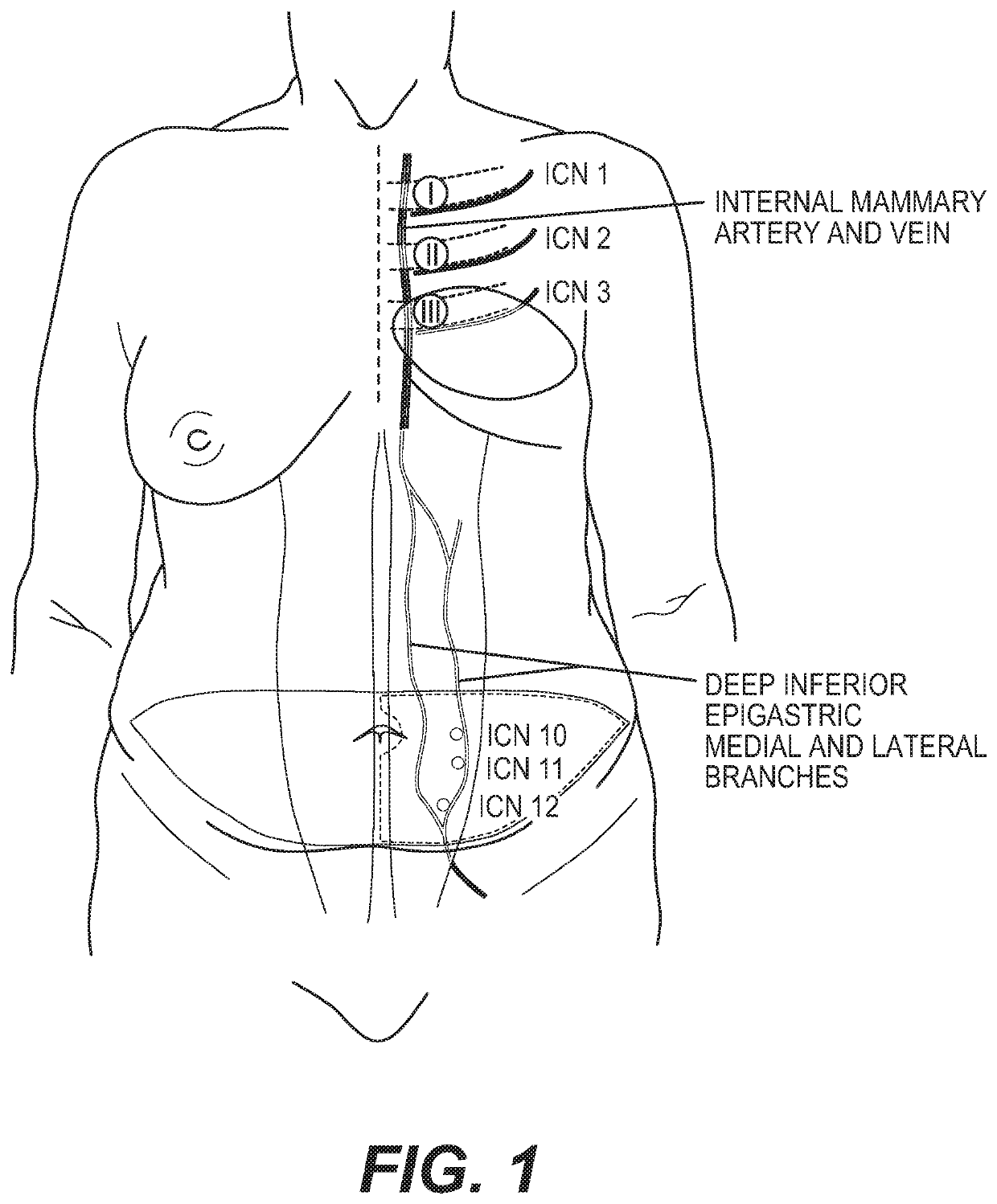
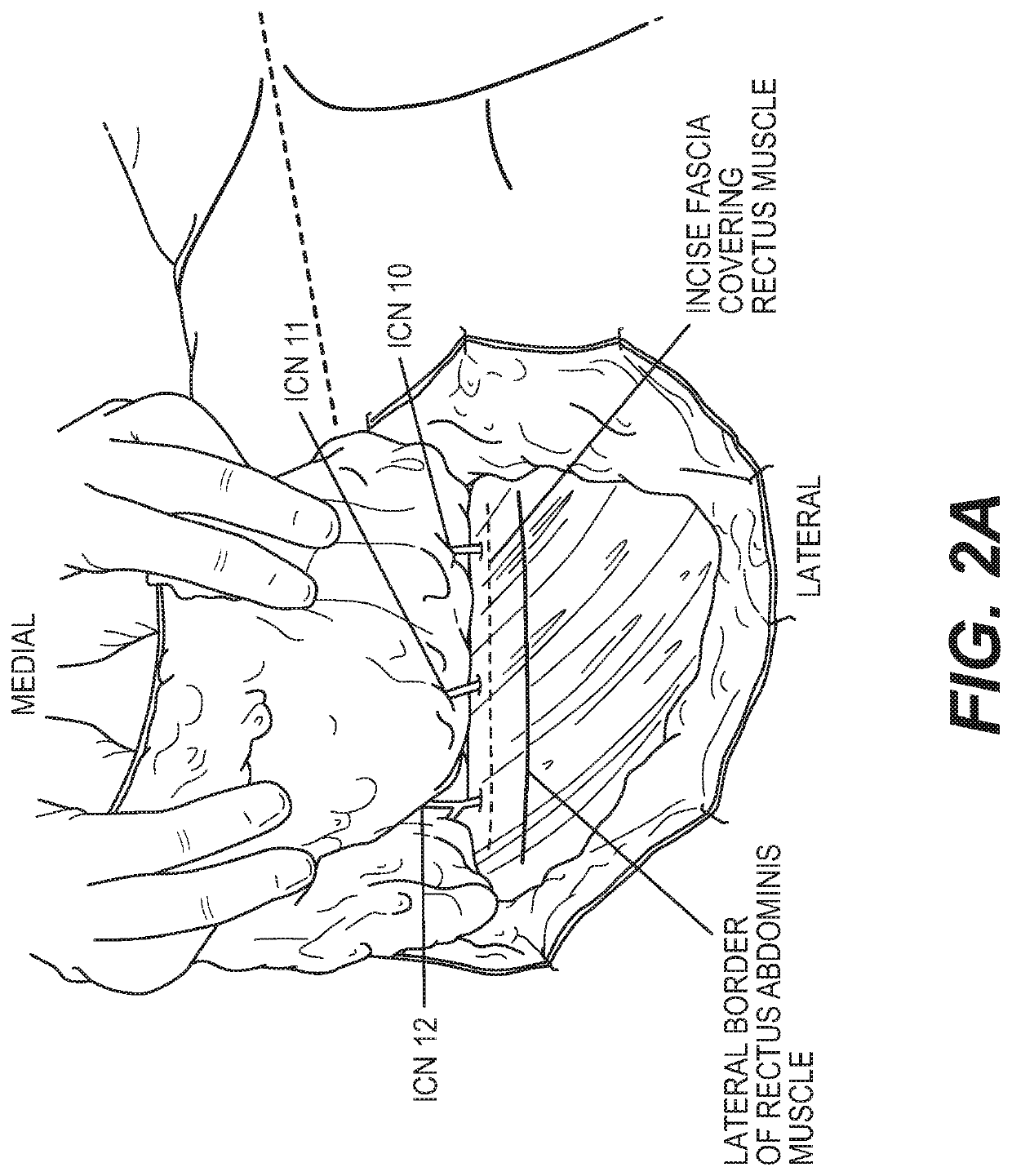
The subject invention pertains to materials, including sets of nerve grafts, for performing breast neurotization with xenograft nerves in breast surgeries, such as reconstructive breast surgery. Certain embodiments of the set of nerve grafts comprise at least two nerve grafts prepared from one or more nerves, such as one or more intercostal nerves (ICNs), obtained from one or more animal sources. Such animal-sourced nerve grafts may be used as xenografts in the reconstruction of nerve defects in humans, and in particular, animal-sourced ICN grafts may be used as xenografts in the reconstruction of ICN nerve defects in humans, including through use of the breast neurotization technique described herein. These animal-sourced nerve grafts may also be used in the reconstruction of nerve defects in animal recipients, including as xenografts, allografts and autografts.
Owner:AXOGEN CORP
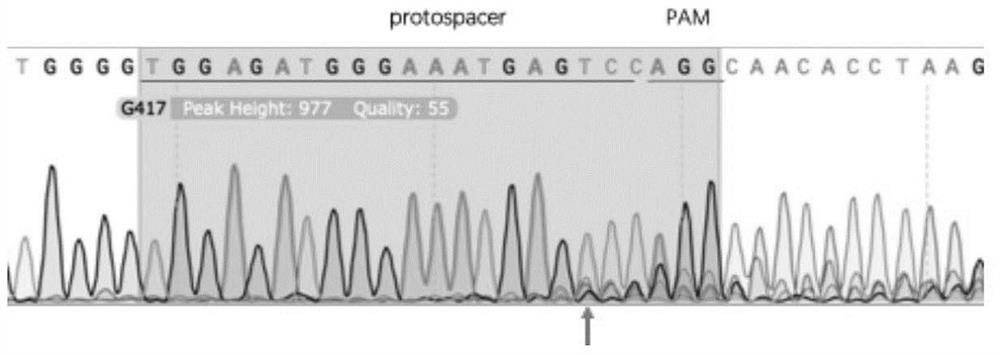
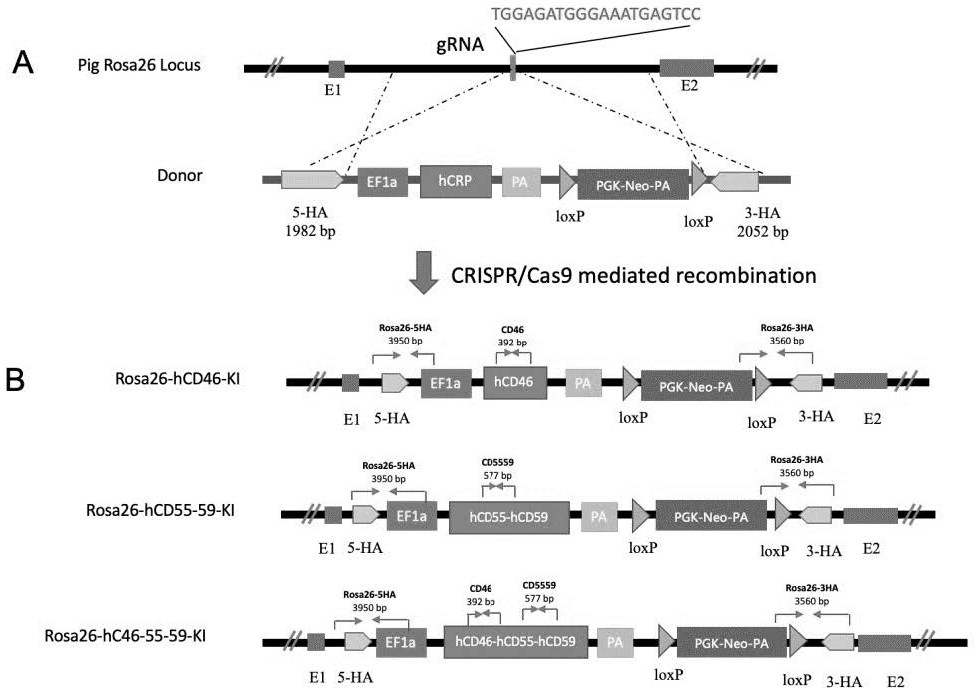
Preparation method of miniature pig with human complement regulatory protein knocked into Rosa26 site at fixed point
ActiveCN114231533AAvoid unclear genetic backgroundAvoid separabilityHydrolasesStable introduction of DNABiotechnologyDisease
The invention discloses a preparation method of a miniature pig with a human complement regulatory protein knocked into a Rosa26 site at a fixed point, which is characterized in that a human complement regulatory protein gene is inserted into a pig ROSA26 gene site in a fixed-point gene recombination mode, so that the miniature pig with constitutive expression of the human complement regulatory protein is constructed. The constructed miniature pig not only avoids the unclear genetic background caused by exogenous random insertion and the unstable expression quantity caused by chromosome separation in the progeny breeding process, but also provides important animal resources for the research of xenotransplantation and immune related diseases in the future, and has important clinical application value.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Farrow skin prosoma organization for renovating skin wound, preparing method and use method thereof
The present invention provides a fetal pig skin precursor tissue for repairing the skin wound. The fetal pig skin precursor tissue is originated from the inbred strain of all pig species, the fetal pig of the blocked group and the fetal pig of the hybridization breeds. The gestational age of the fetal pig is from 21 days till the birth day, which comprises direct obtaining the fetal pig skin precursor tissue or the fetal pig skin precursor tissue after gene modification. The xenotransplantation is conducted after preparing the tissue into the shape of the cell suspension, the cell mass, the particle or the stamp skin or the big piece of skin. Under proper microenvironment, the present invention comprises the cleaning, the low cell immune response ability, induction of the immune tolerance between the host and the implant (fetal pig skin) or modification in the pig skin precursor tissue. The skin precursor tissue provided in the present invention can grow and develop into a complete skin with the dermis, the epidermis and the appendages of the skin. The present invention can be used in repairing the skin defect caused by various kinds of reasons. The present invention can promote the wound healing; therefore a complete skin is formed.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com