Cell culture method
A cell culture and nuclear cell technology, applied in cell culture active agent, culture process, tissue culture and other directions, can solve the problems of high cost, poor activity, low purity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Prepare activation medium and proliferation medium, the steps are as follows:
[0043] 1. Preparation of activated medium
[0044]Serum-free RPMI1640 medium was used as the basal medium, and anti-CD16 monoclonal antibody, IL-2, ascorbic acid, TGF-β1 and thymosin were added to the basal medium, and mixed evenly to make anti-CD16 monoclonal antibody, IL-2, ascorbic acid The final concentrations of , TGF-β1 and thymosin in the activation medium were 60ng / ml, 250U / ml, 2ng / ml, 60ng / ml and 2ng / ml respectively. Sterilize with a 0.1μm membrane filter, and store in the dark at 4-8°C after aliquoting.
[0045] 2. Preparation of Proliferation Medium
[0046] Serum-free RPMI1640 medium was used as the basal medium, and IL-1α, IL-2, ascorbic acid, IL-21, IL-7 and 41-BBL were added to the basal medium. Mix well so that the final concentrations of IL-1α, IL-2, ascorbic acid, IL-21, IL-7 and 41-BBL in serum-free RPMI1640 medium are 50ng / ml, 500U / ml, 2ng / ml, 60ng / ml, respectively. m...
Embodiment 2
[0049] Cell culture, the steps are as follows:
[0050] Step 1, separating peripheral blood mononuclear cells (PBMC), the steps are as follows:
[0051] 50ml of peripheral blood was collected from healthy people. According to the method reported in the literature, peripheral blood PBMCs were separated using lymphocyte separation medium (purchased from Tianjin Haoyang). Carefully aspirate the cells in the middle cloud layer to separate the peripheral blood mononuclear cells (PBMCs), and wash them twice with normal saline. Add activation medium to resuspend PBMCs, and perform cell counting.
[0052] Step 2, the cultivation of NK cells, the steps are as follows:
[0053] Adjust the PBMC density with the activation medium prepared in Example 1 to be 2×10 6 cells / ml, and 56°C inactivated autologous plasma (plasma collected in step 1 in Example 2) was added, so that the volume percentage of autologous plasma in the activation medium was 4%. Transfer the PBMC NK cells and their m...
Embodiment 3
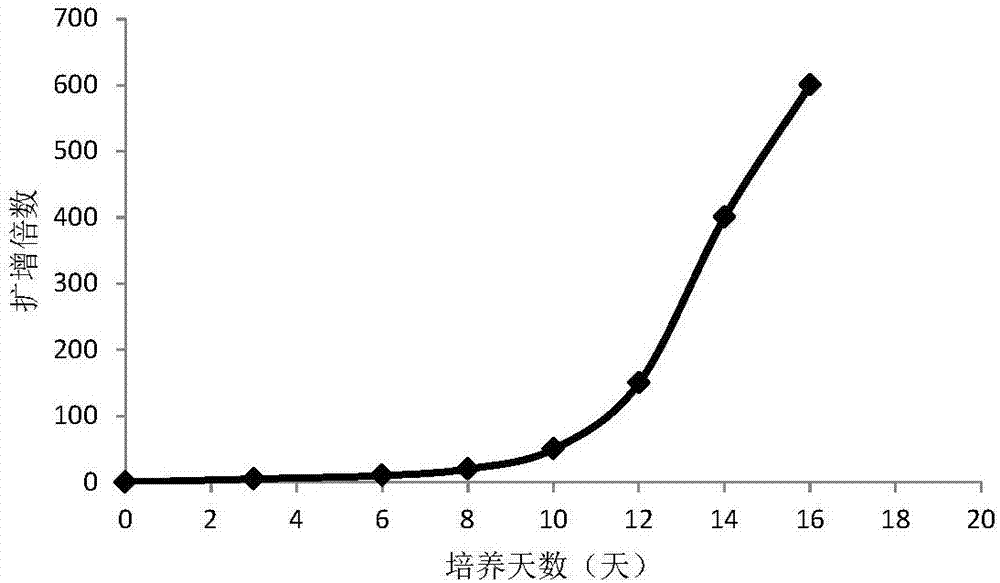
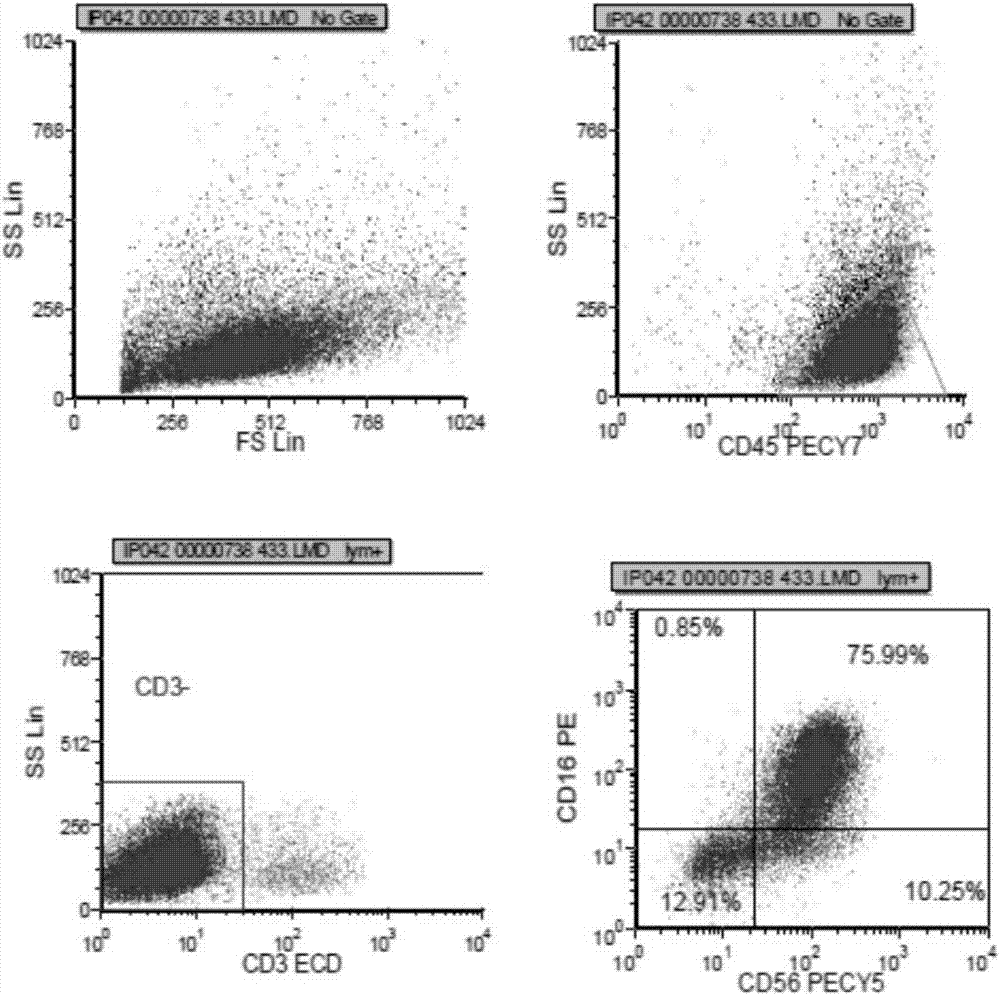
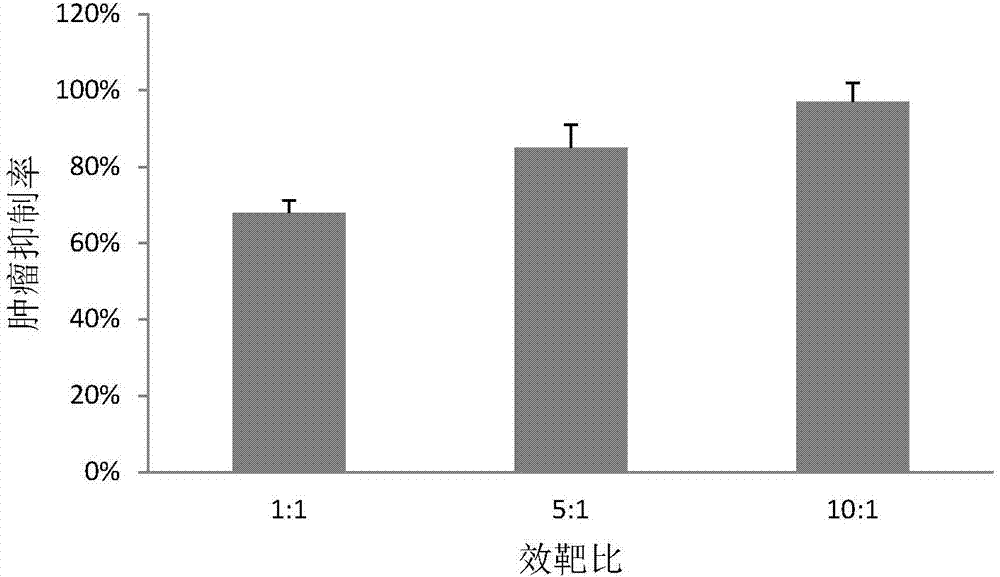
[0057] The peripheral blood mononuclear cells cultured to the 16th day were collected, and the ratio of CD3-CD56+CD16+ cells was detected by flow cytometry. figure 2 It is the flow cytometric detection result of NK cells on the 16th day obtained by adopting the method of the present invention. As shown in the results, the ratio of CD3-CD56+CD16+ of peripheral blood mononuclear cells obtained by the present invention reaches 75%, indicating that through 16 days of cultivation, the purity of NK cells (NK cells accounting for the percentage of total cells) cultivated in vitro is as high as 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com