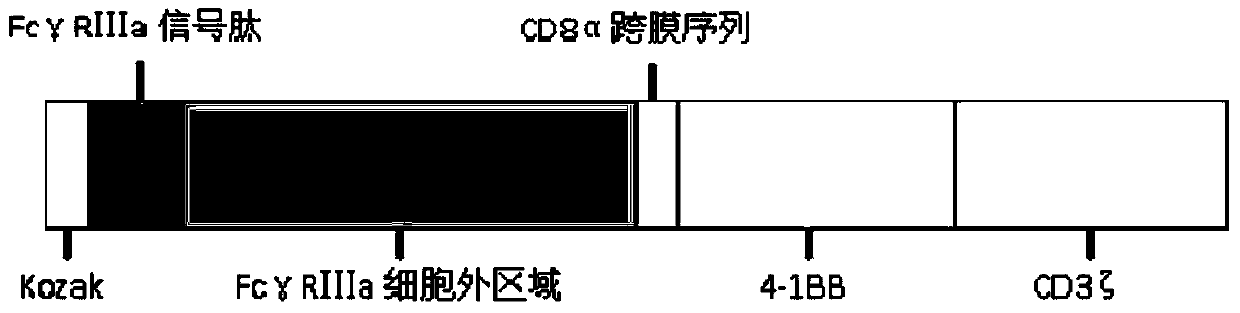
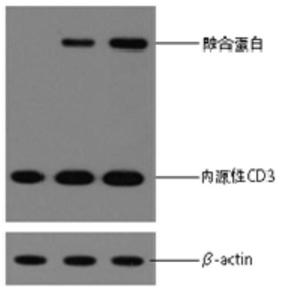
A chimeric gene based on fcγriiia and its use
A chimeric gene, coding gene technology, applied in the field of genetically engineered immune cells based on FcγRIIIa, can solve the problems of lack of versatility and limit the clinical application of CAR technology, and achieve the effect of improving clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1F
[0075] Example 1 Identification of Restriction Endonuclease BamH / Sal1 Double Digestion of FcγRⅢa-BB-ζ Lentiviral Expression Vector
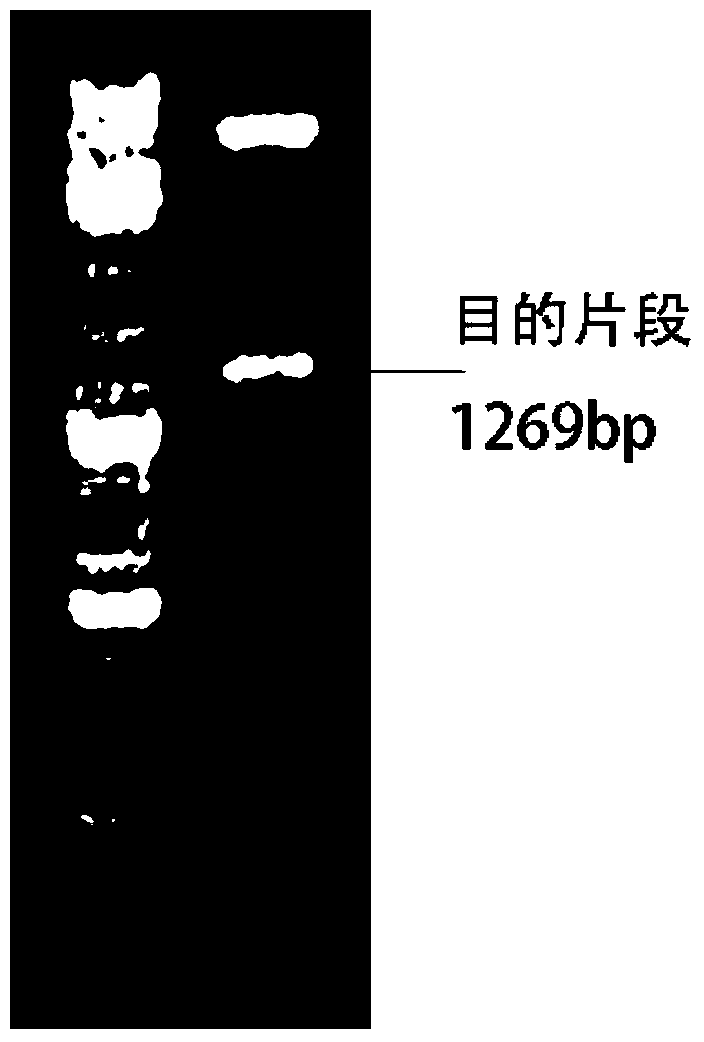
[0076] figure 2 It is the part of the target gene released after the chimeric gene FcγRⅢa-BB-ζ is digested with BamH1 and Sal1 on the lentiviral expression vector pLVX-EF1α vector, and the target gene fragment is 1269bp. Swimming lane 1 is the nucleic acid molecular weight standard, and swimming lane 2 is the double digestion product of BamH1 and Sal1.
[0077] The correct plasmid identified by double enzyme digestion was sent to Shanghai Sangon Bioengineering Co., Ltd. to sequence the inserted chimeric gene fragment. The correct plasmid was named pLVX-FcγRⅢa-BB-ζ.
Embodiment 2F
[0078] Example 2 Preparation of FcγRⅢa-BB-ζ lentivirus
[0079] The FcγRIIIa-BB-ζ chimeric gene was designed and constructed as described herein. The lentivirus preparation and purification of the chimeric gene FcγRⅢa-BB-ζ adopts the following method:
[0080] 1. Use the Qiagen EndoFree Plasmid Maxi Kit to extract the plasmid;
[0081] 2. Prepare 293T cells in 100mm culture dish in advance;
[0082] 3. After digesting the cells with trypsin, draw 10ml of complete medium with an electric pipette, pipette all the cells into a single-cell suspension, and transfer them to other 100mm culture dishes in proportion; 37°C, 5% CO 2 incubator overnight;
[0083] 4. Transfect when the cell confluency is 70%-80%;
[0084] 5. Replace the cell medium and replace the whole amount with fresh serum-free DMEM medium;
[0085] 6. Prepare plasmid mixture: take a new 1.5ml centrifuge tube, add 0.5mL DMEM and plasmid;
[0086] 7. Prepare PEI mixture: take a new 1.5ml centrifuge tube, add 0.5m...
Embodiment 3
[0102] Example 3 Preparation of cell product of chimeric FcγRⅢa-BB-ζ gene
[0103] The cells come from patients or healthy donors, and peripheral blood mononuclear cells (PBMC) are obtained by venous blood sampling or blood component apheresis. The method of T cell culture adopts CD3, CD28 monoclonal antibody coating culture flask to activate T cell method, or adopts the paramagnetic polypropylene beads T cell activation method coated with CD3 and CD28 monoclonal antibody, NK cell culture method is carried out as described (Hiroyuki et al., Cancer Res 2009;69:4010-4017). Lentiviral transduction was performed as described (Levine et al., 2006, Proc Natl Acad Sci USA 103:17372-17377).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com