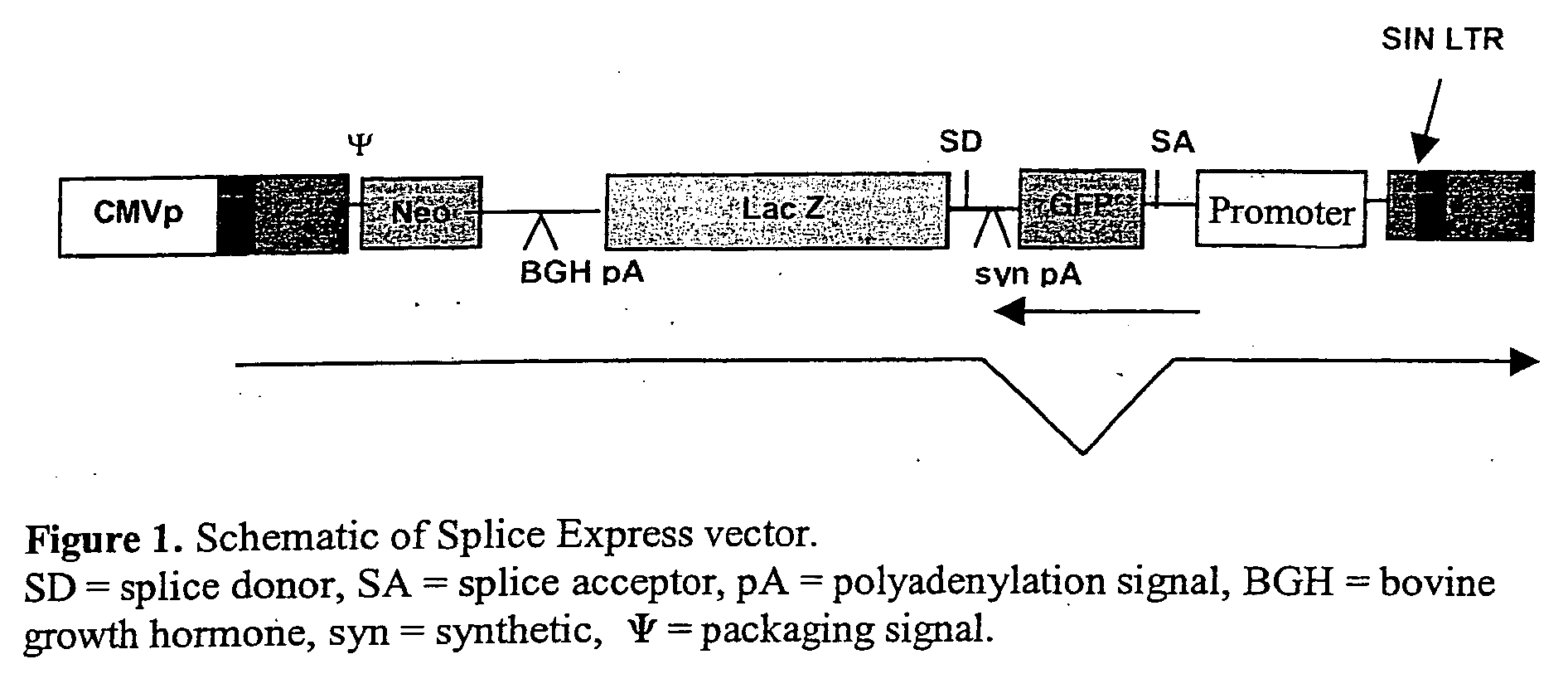
Patents
Literature
6283results about "Reverse transcribing RNA viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
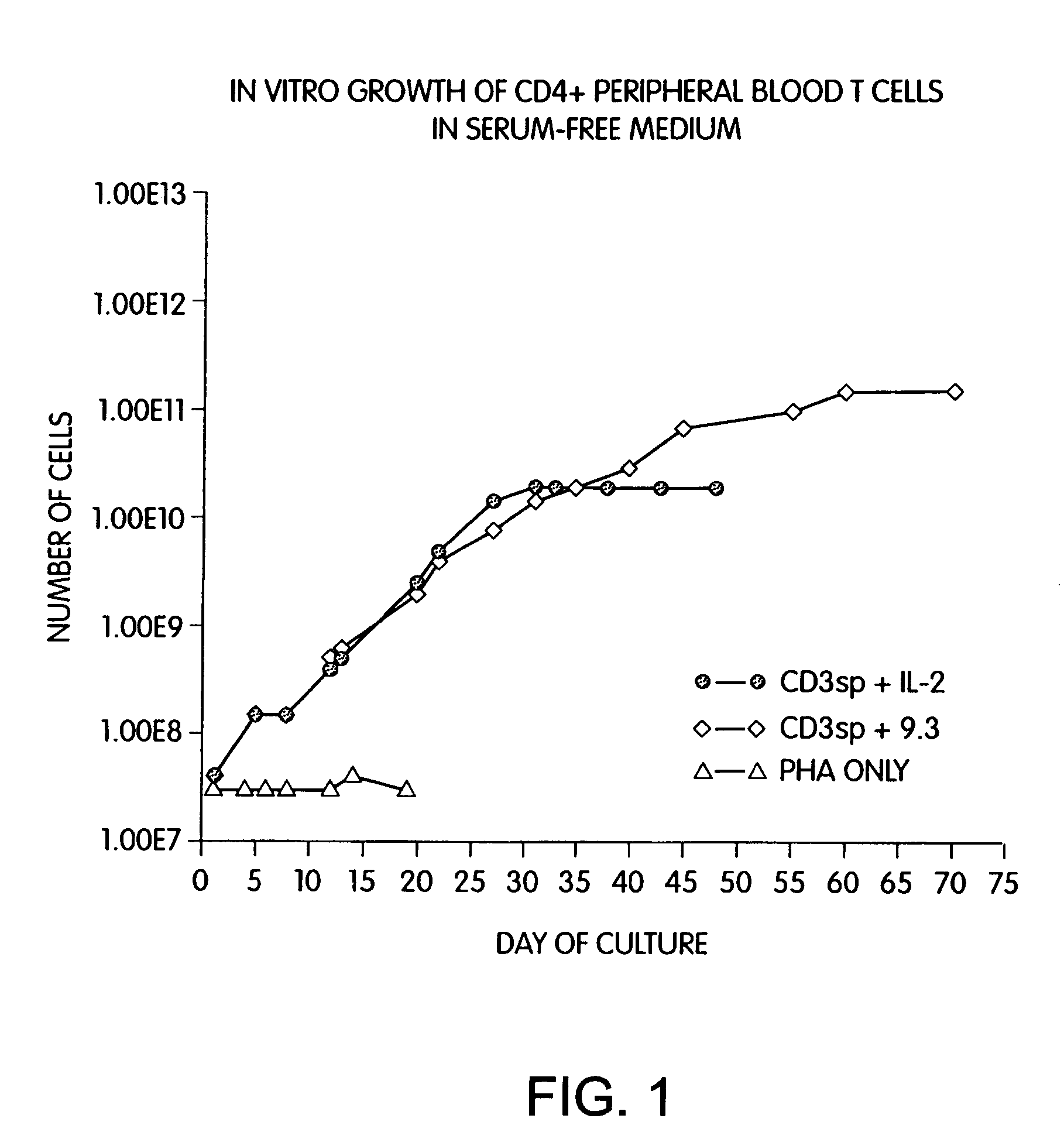
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
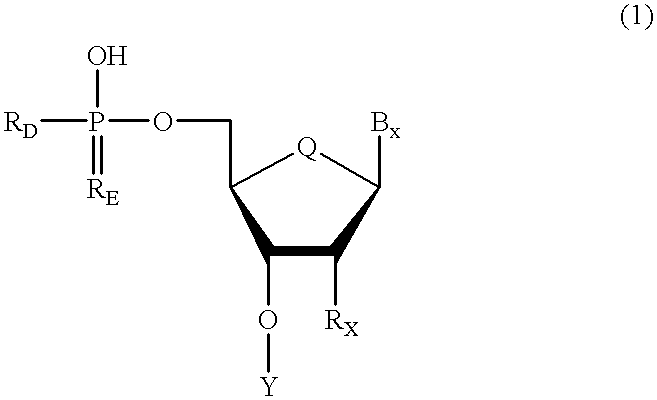
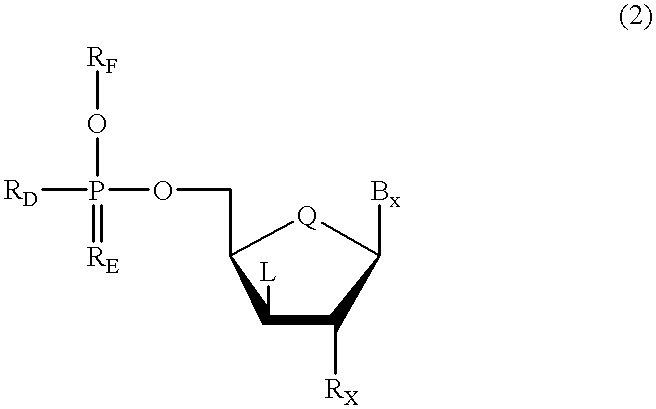
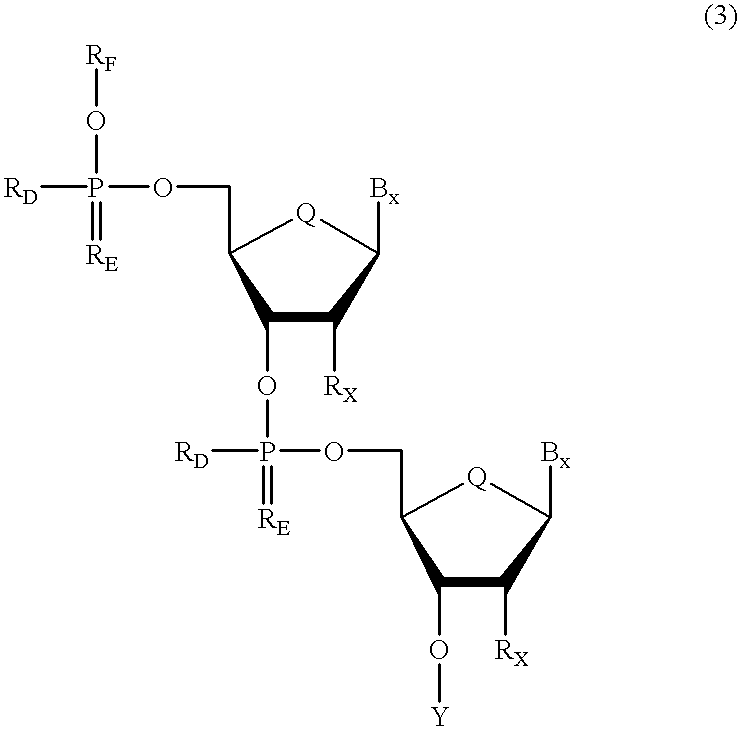
Oligonucleotides having chiral phosphorus linkages
InactiveUS6239265B1Improved pharmacokinetic propertiesImprove propertiesPeptide/protein ingredientsGenetic material ingredientsSugar moietyPhosphoramidate
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
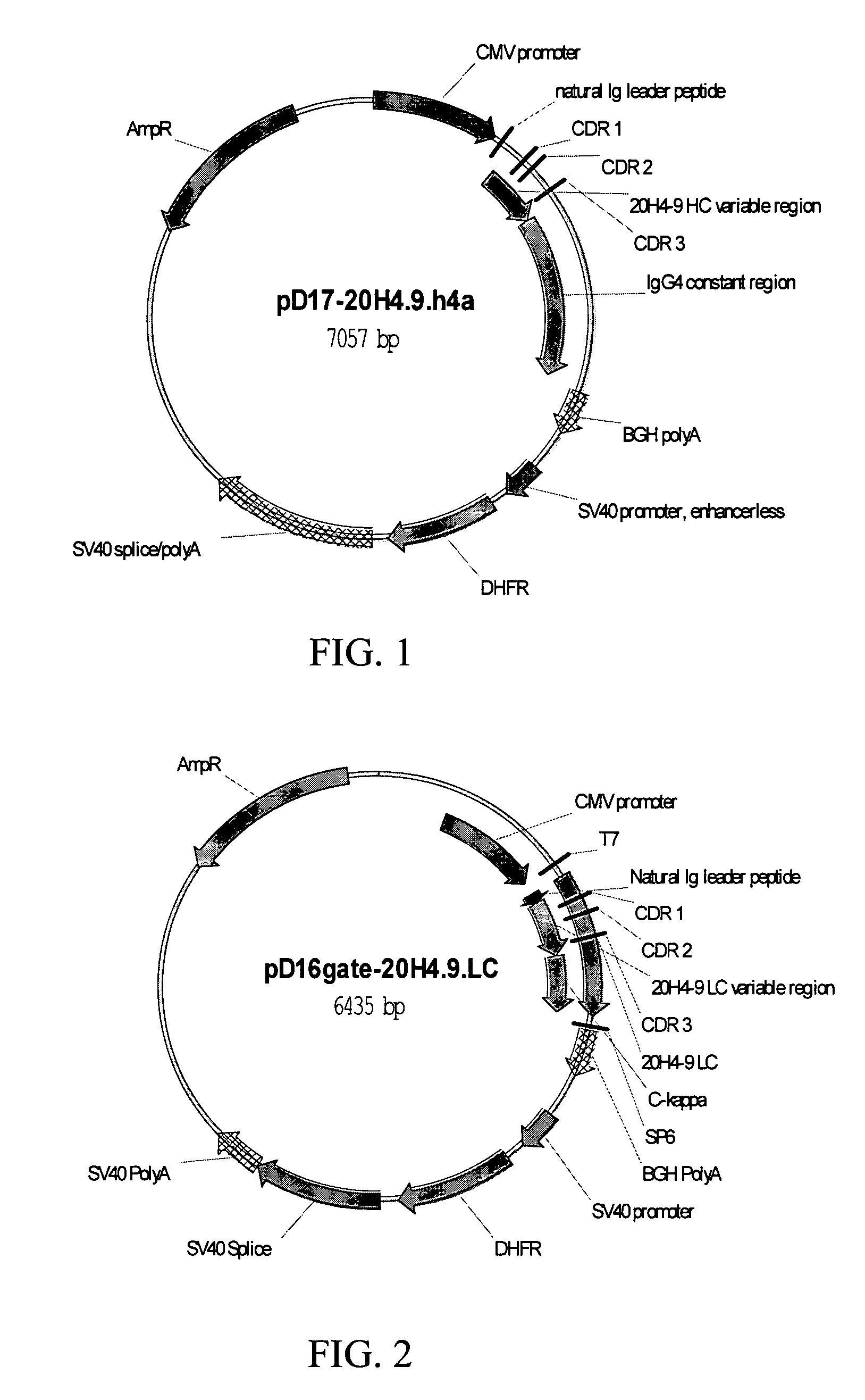
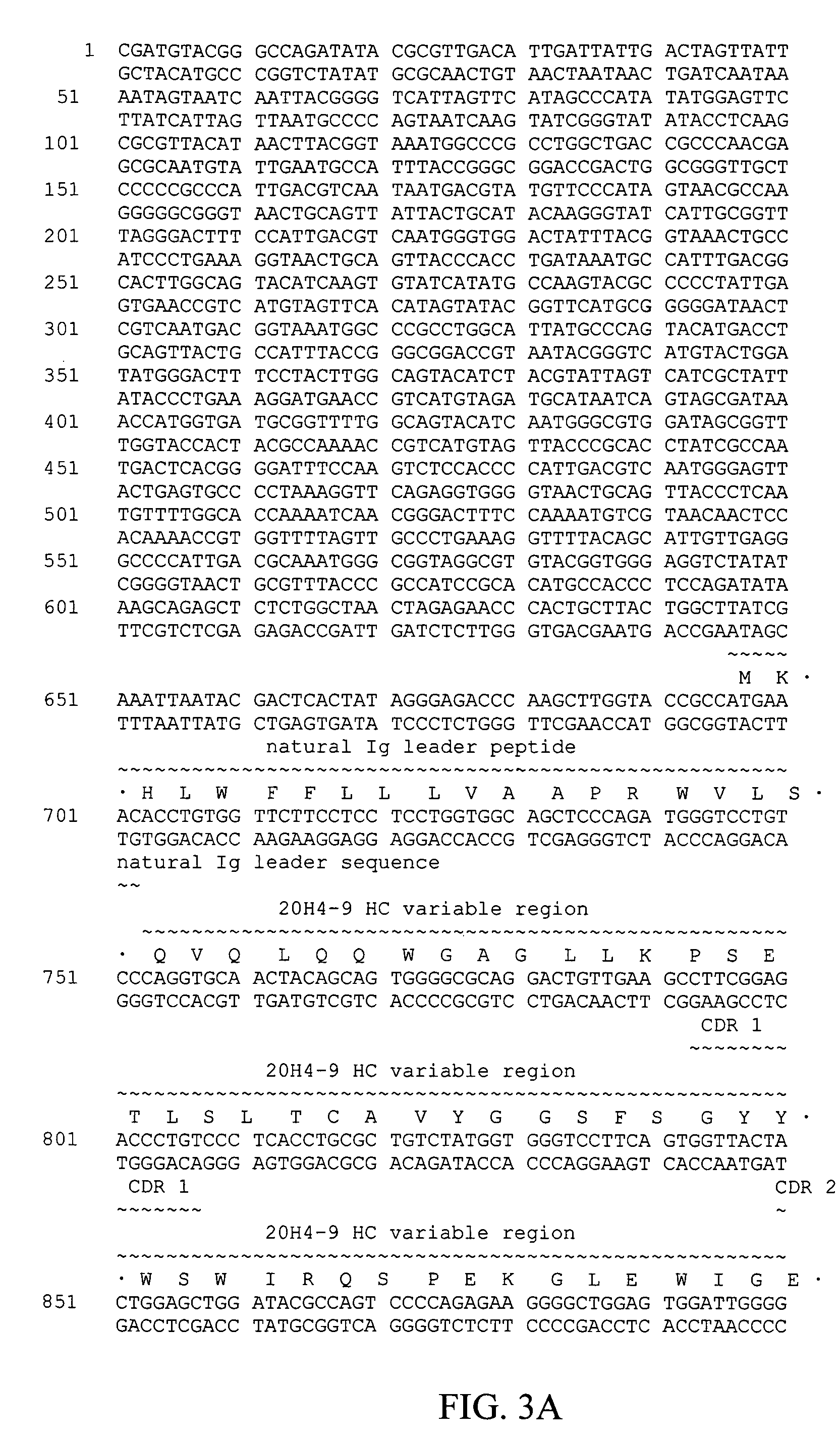
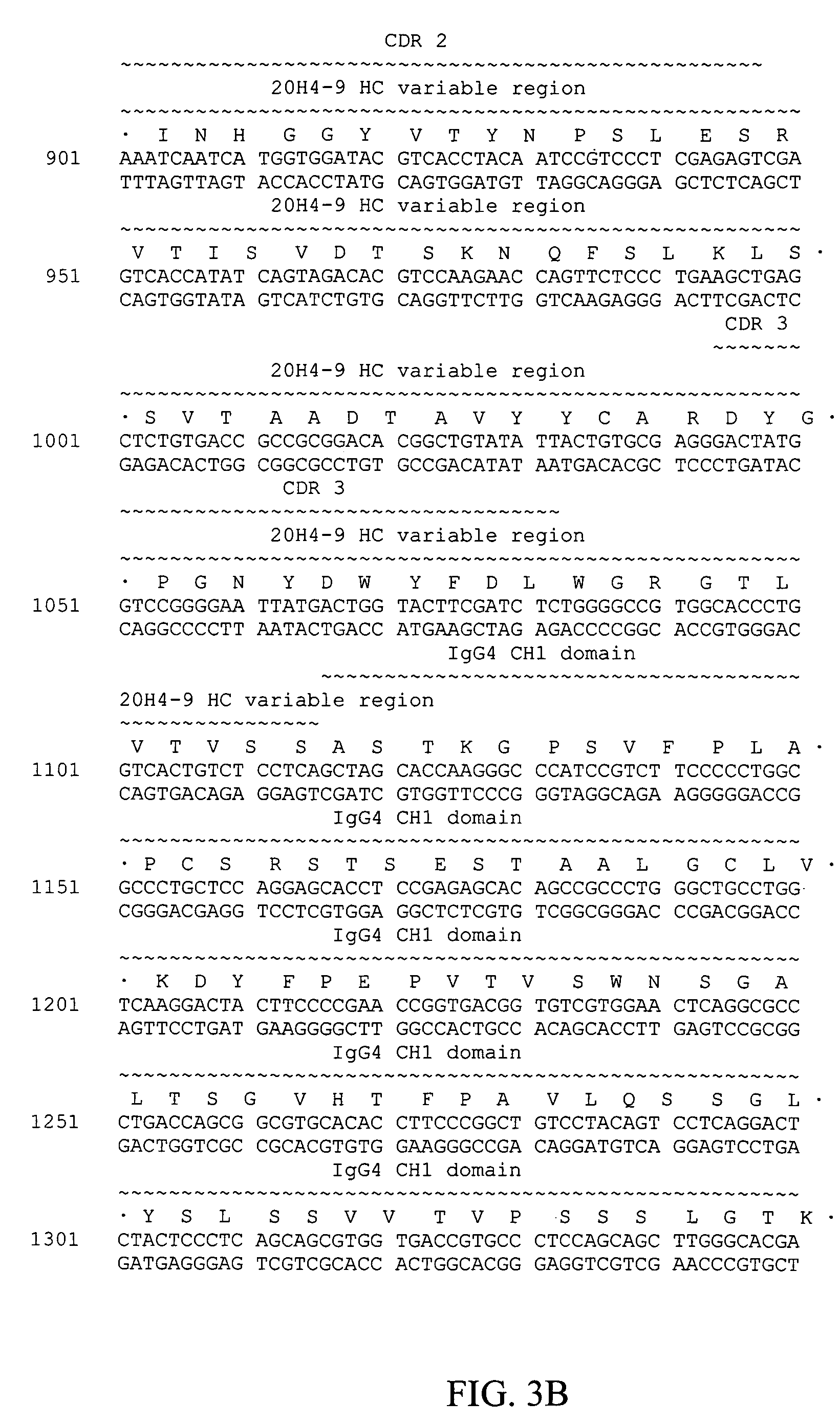
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
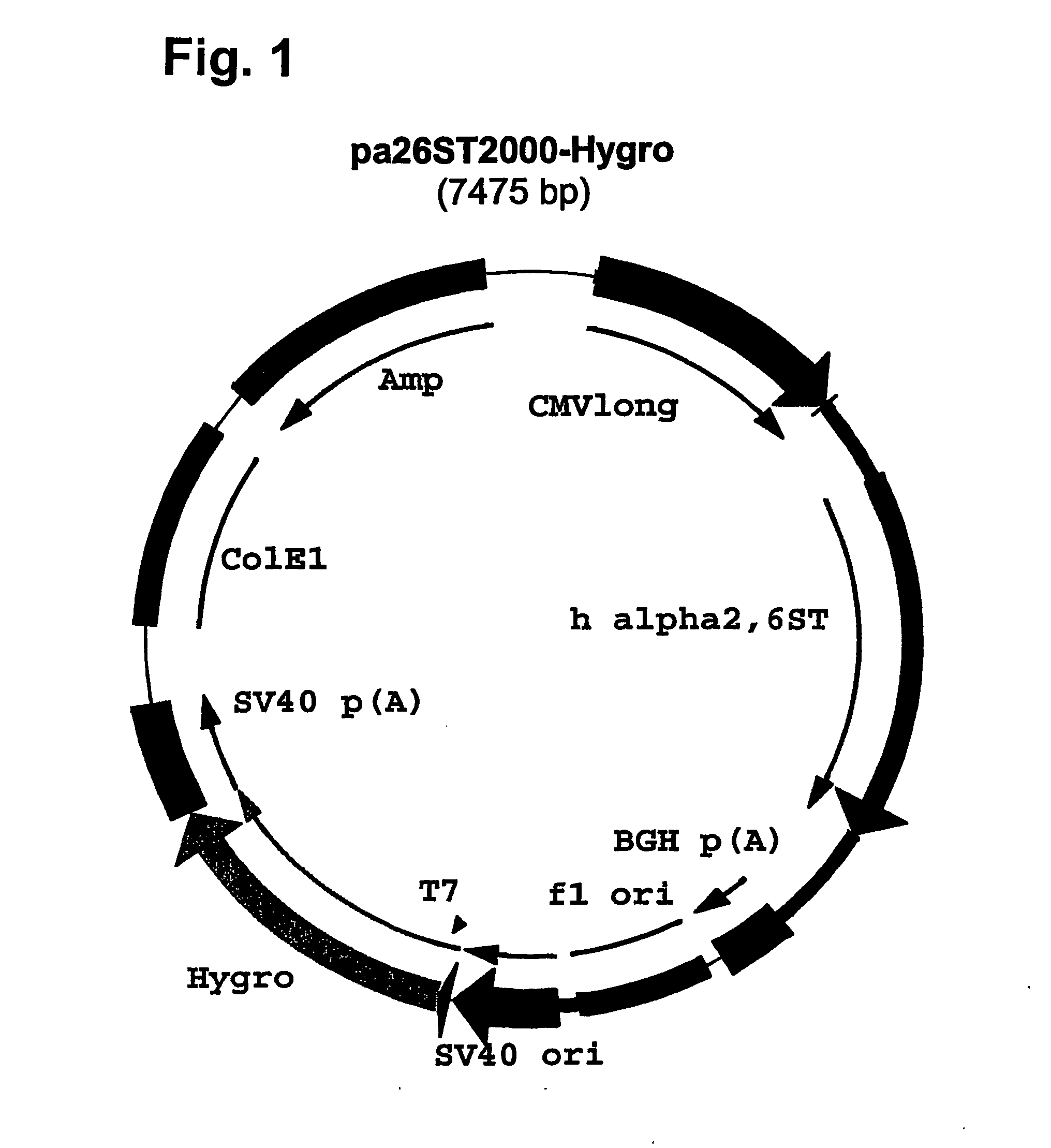
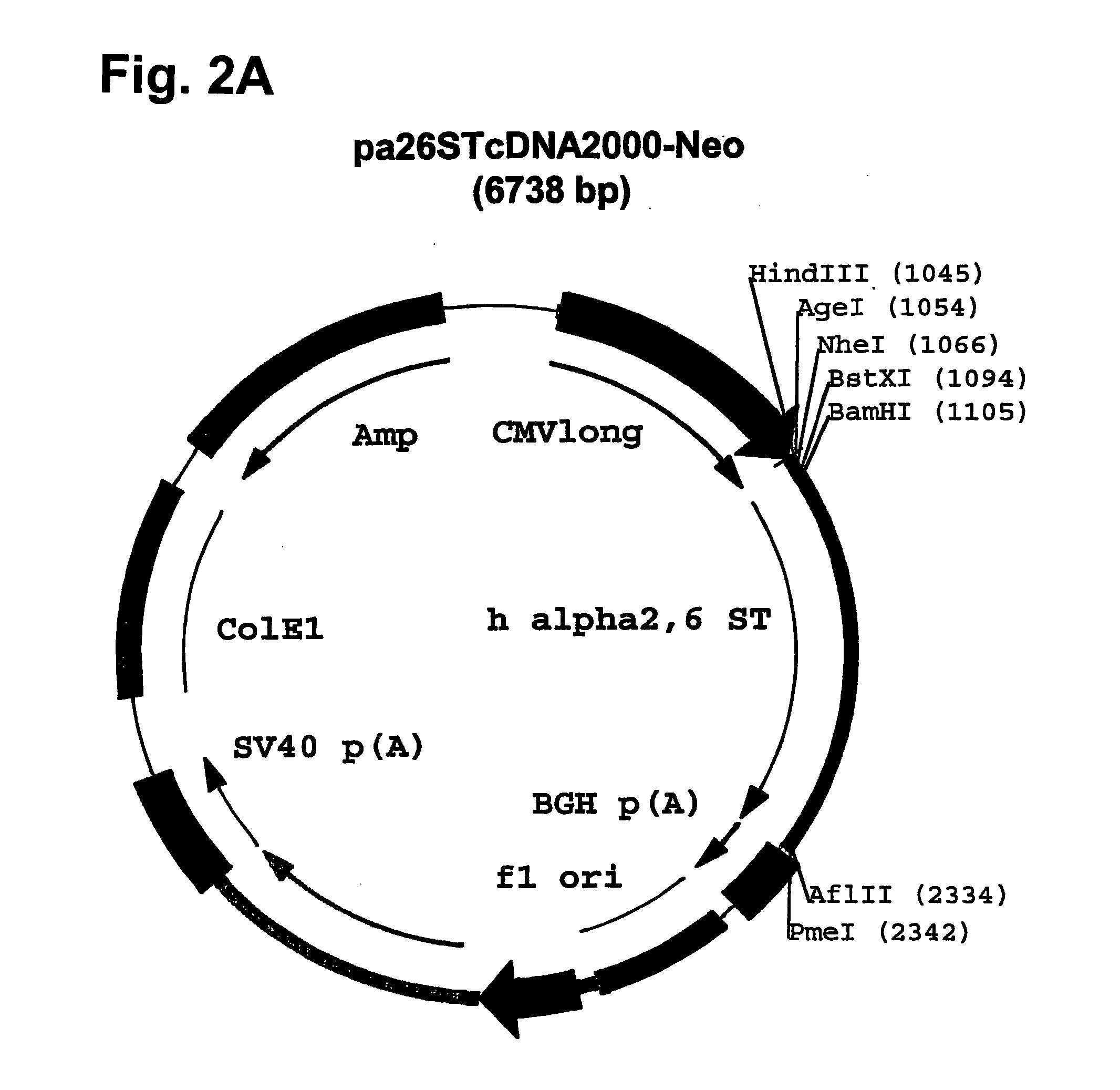
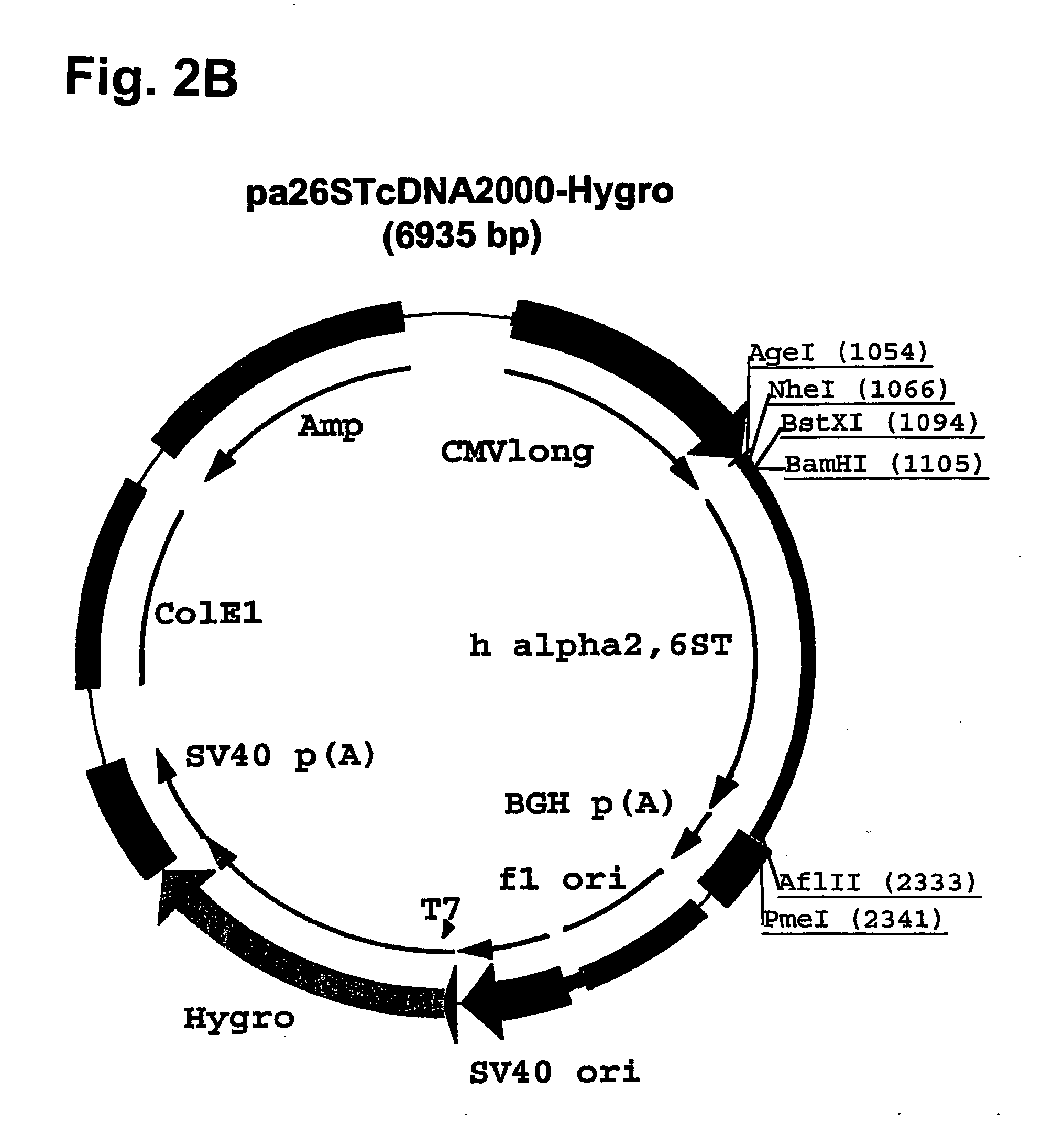
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Method of eliminating inhibitory/instability regions from mRNA
InactiveUS6174666B1Microbiological testing/measurementVirus peptidesImmunodeficiency virusInstability
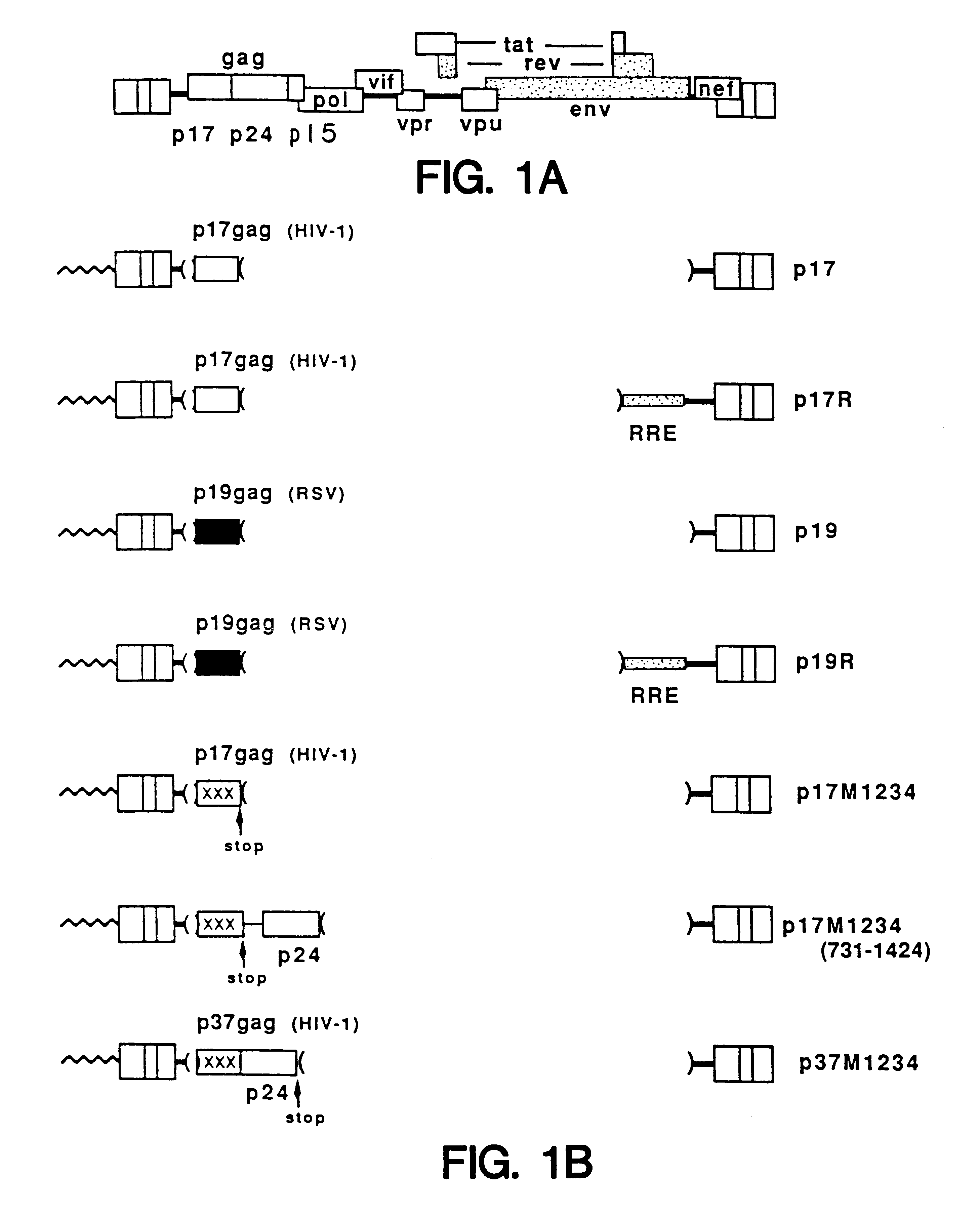
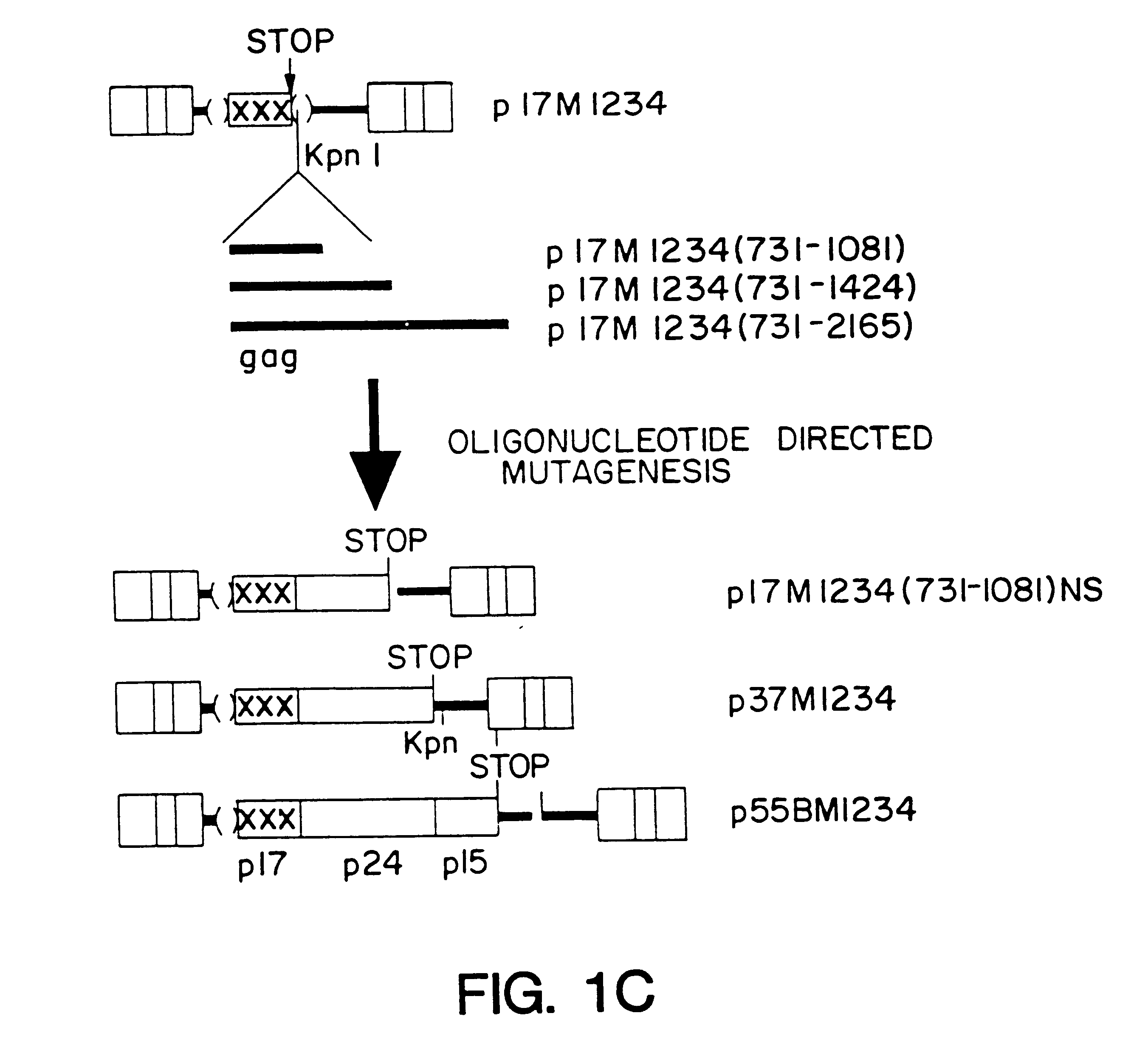
A method of locating an inhibitory / instability sequence or sequences within the coding region of an mRNA and modifying the gene encoding that mRNA to remove these inhibitory / instability sequences by making clustered nucleotide substitutions without altering the coding capacity of the gene is disclosed. Constructs containing these mutated genes and host cells containing these constructs are also disclosed. The method and constructs are exemplified by the mutation of a Human Immunodeficiency Virus-1 Rev-dependent gag gene to a Rev-independent gag gene. Constructs useful in locating inhibitory / instability sequences within either the coding region or the 3' untranslated region of an mRNA are also disclosed.
Owner:UNITED STATES OF AMERICA
Microparticles with adsorbent surfaces, methods of making same, and uses thereof
InactiveUS6884435B1Stimulate immune responseEasy to usePowder deliverySsRNA viruses positive-senseAntigenDisease
The present invention is directed to microparticles, to microparticle compositions containing the same, to methods of forming the same, and to uses for the same, including use for a vaccine, for raising an immune response, for treatment of a disease and for diagnosis of a disease. The microparticles comprise a biodegradable polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, or a polycyanoacrylate, and a detergent selected from a cationic detergent and an anionic detergent. The microparticles further comprise an antigen adsorbed on the surface of the microparticle.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
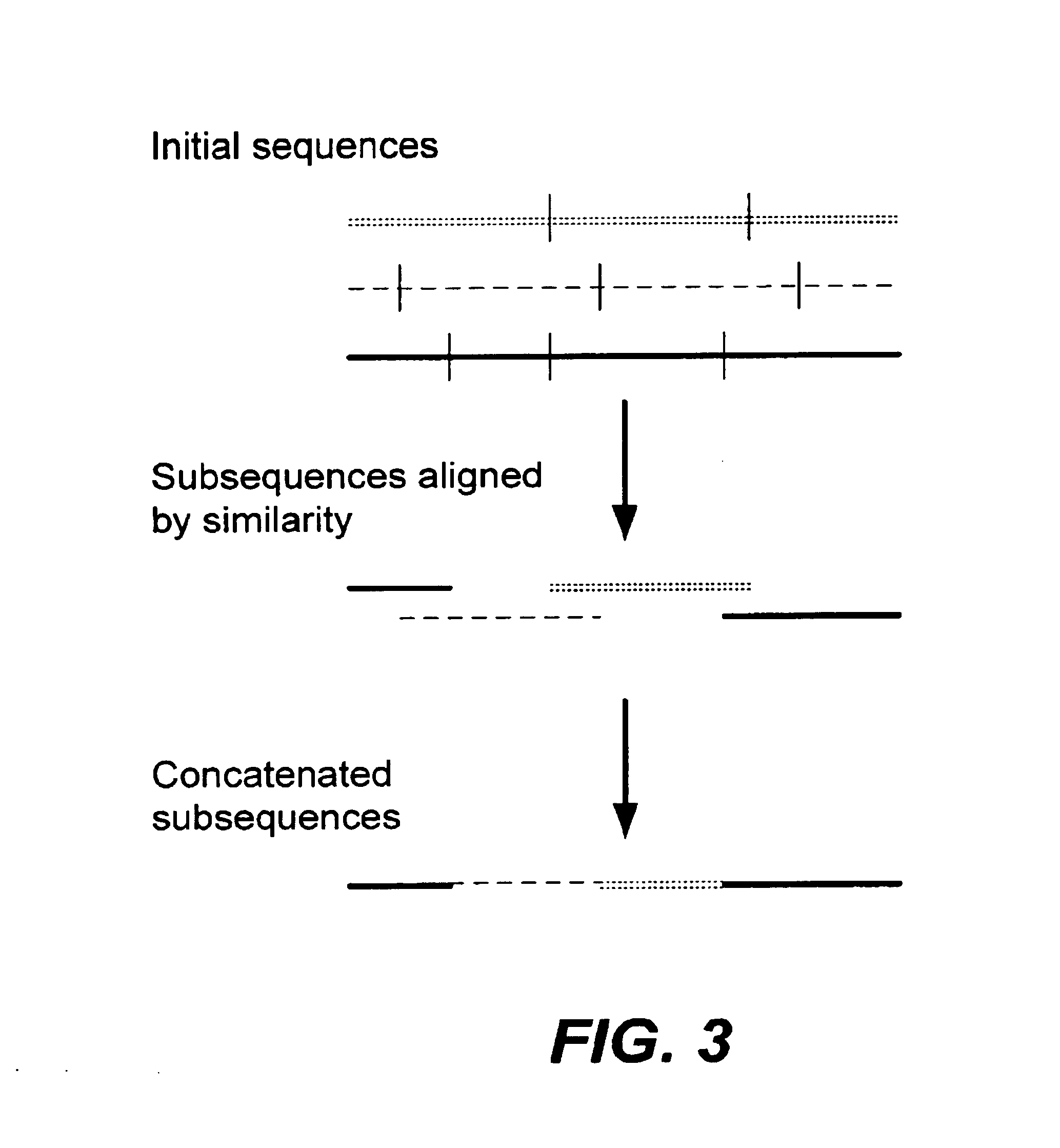
Methods of populating data structures for use in evolutionary simulations
In particular, this invention provides novel methods of populating data structures for use in evolutionary modeling. In particular, this invention provides methods of populating a data structure with a plurality of character strings. The methods involve encoding two or more a biological molecules into character strings to provide a collection of two or more different initial character strings; selecting at least two substrings from the pool of character strings; concatenating the substrings to form one or more product strings about the same length as one or more of the initial character strings; adding the product strings to a collection of strings; and optionally repeating this process using one or more of the product strings as an initial string in the collection of initial character strings.
Owner:CODEXIS MAYFLOWER HLDG LLC
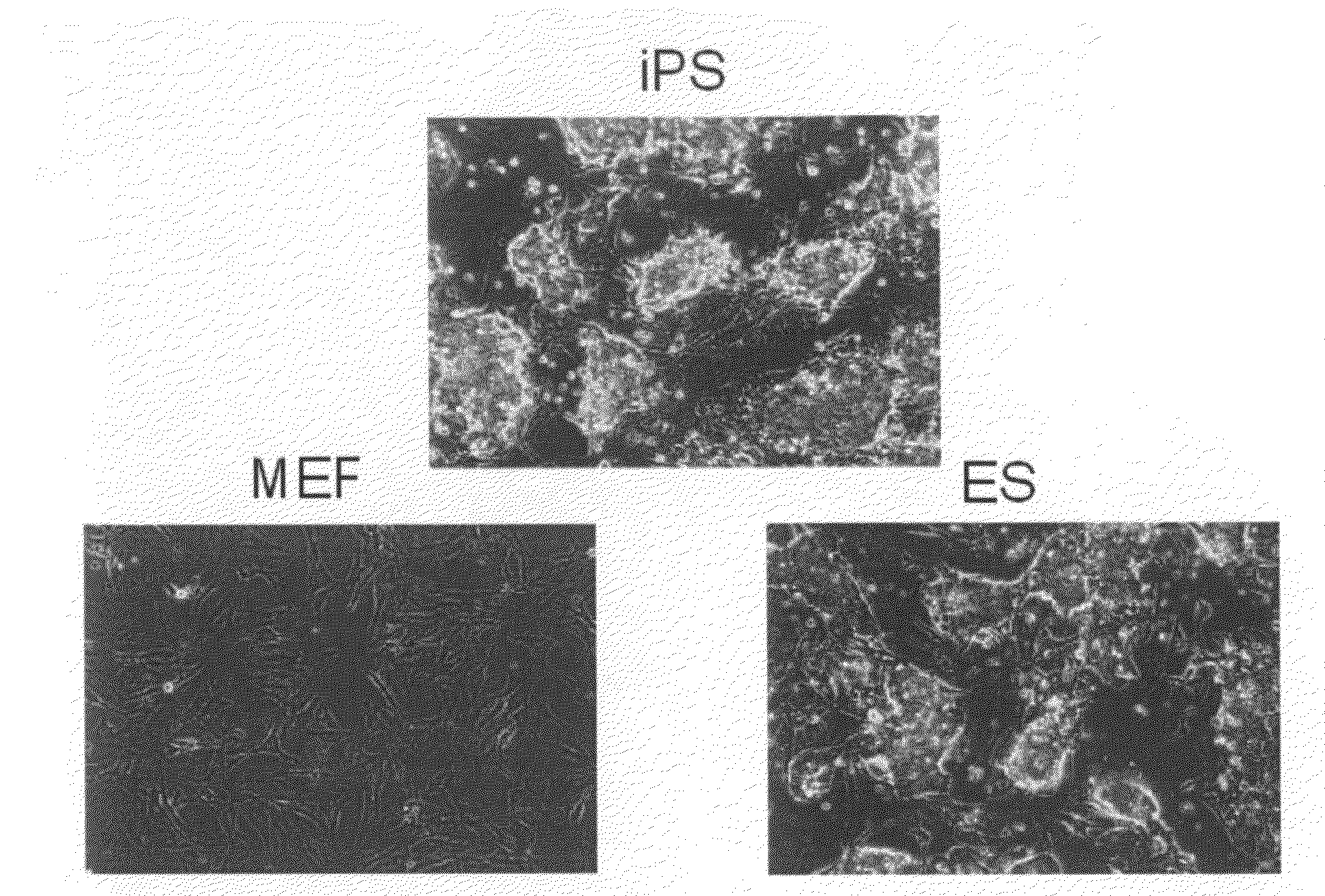
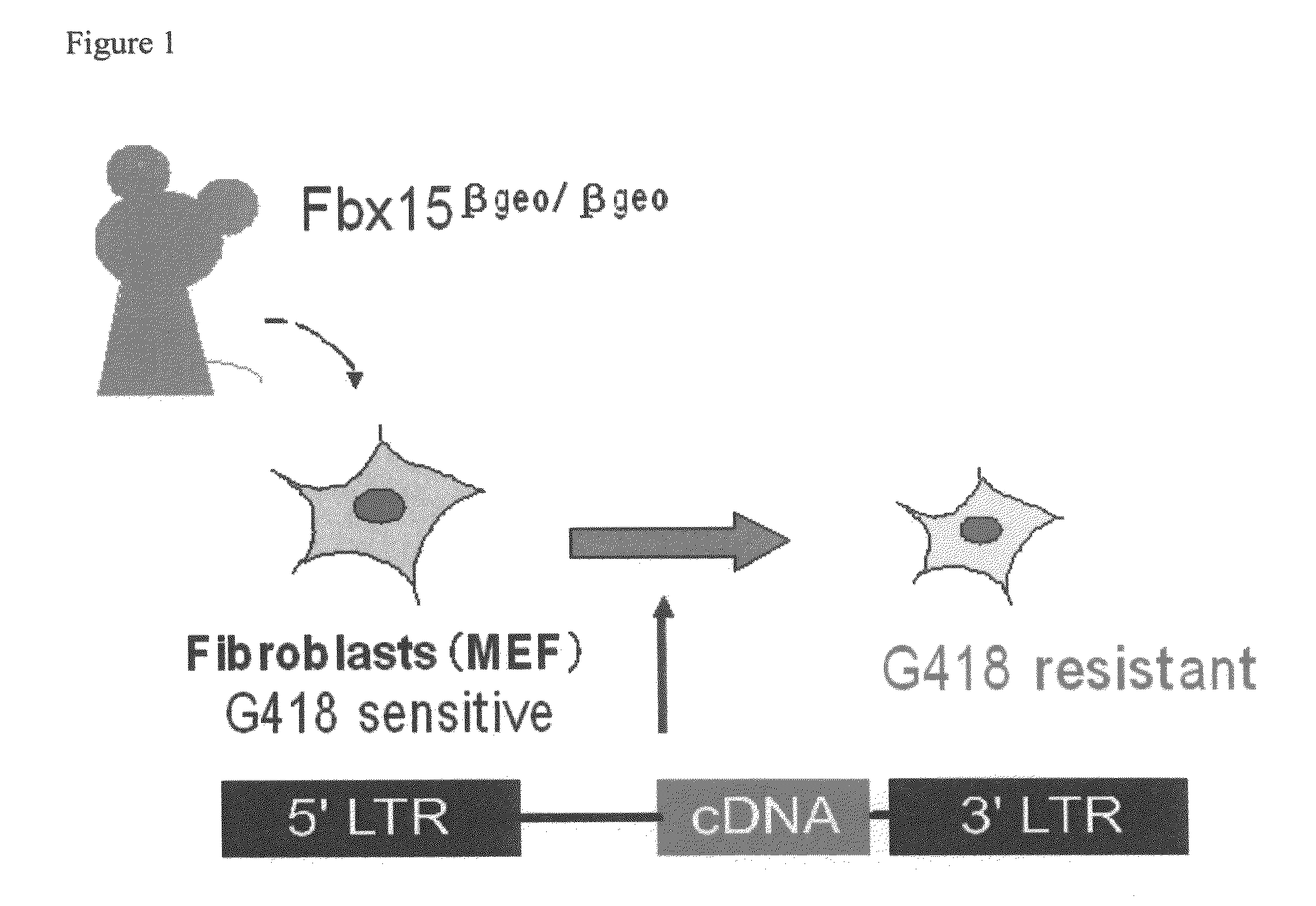
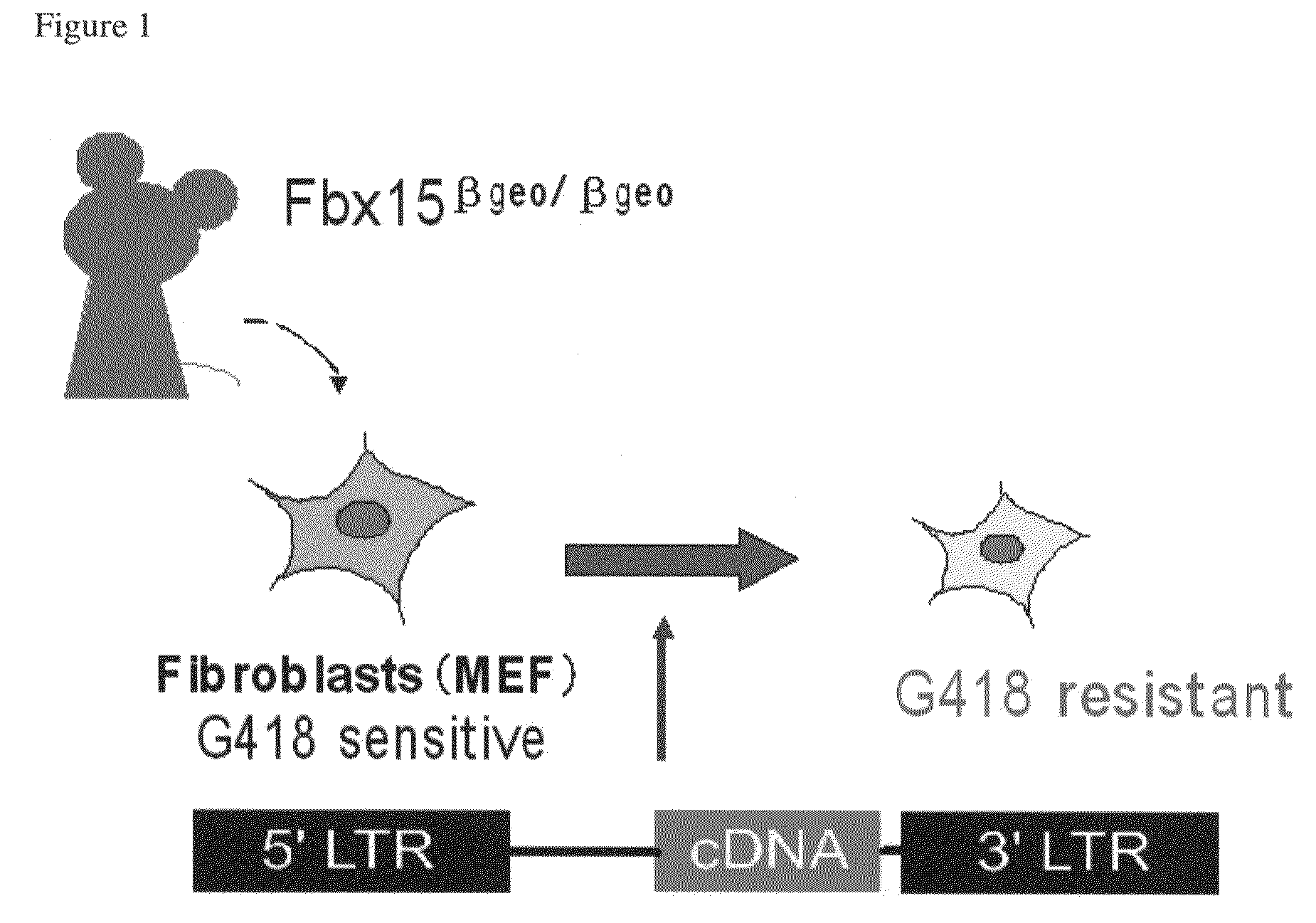
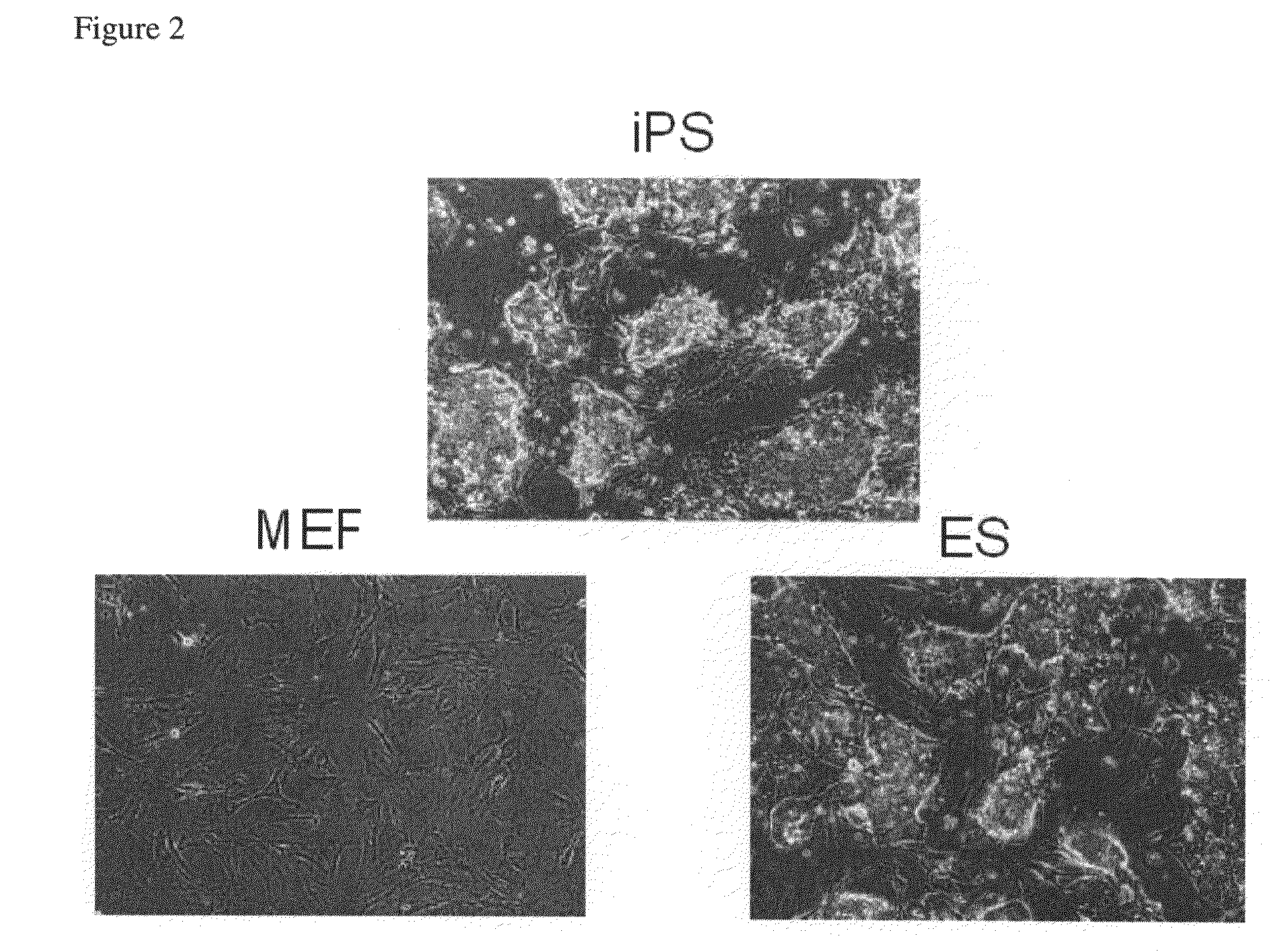
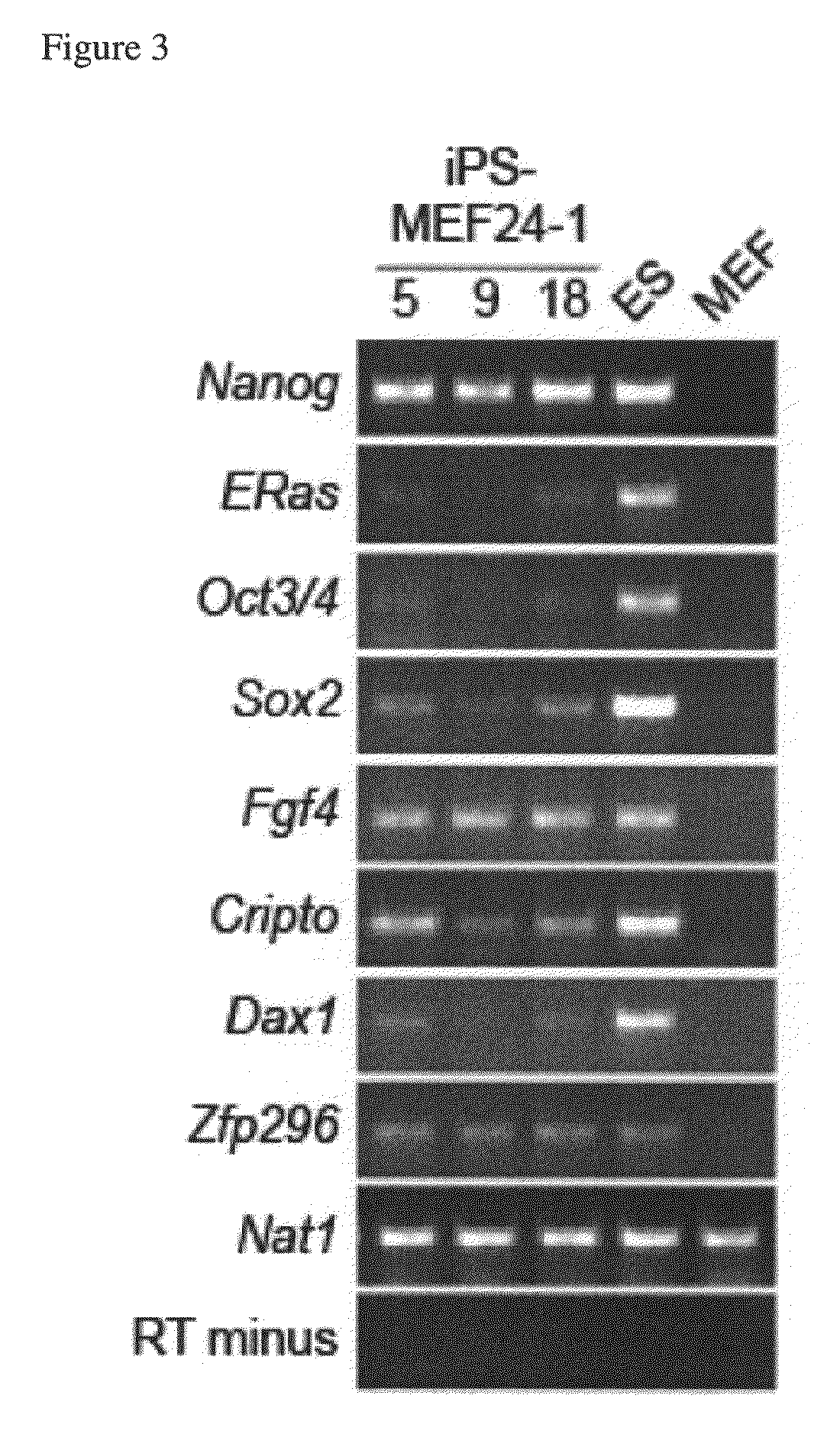
Nuclear reprogramming factor and induced pluripotent stem cells
InactiveUS20090227032A1Easy to prepareEffective isolationGenetically modified cellsPeptidesNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
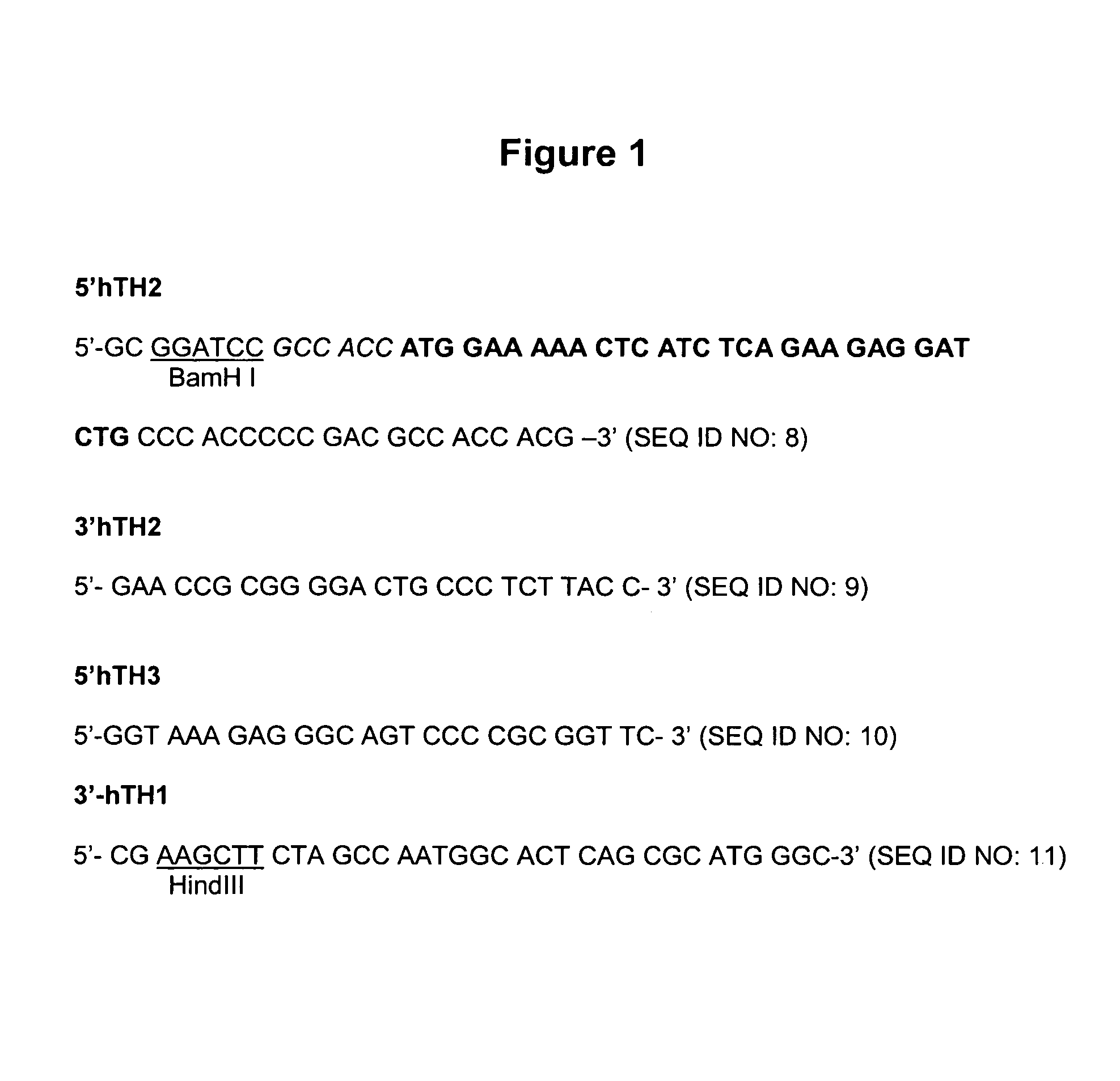
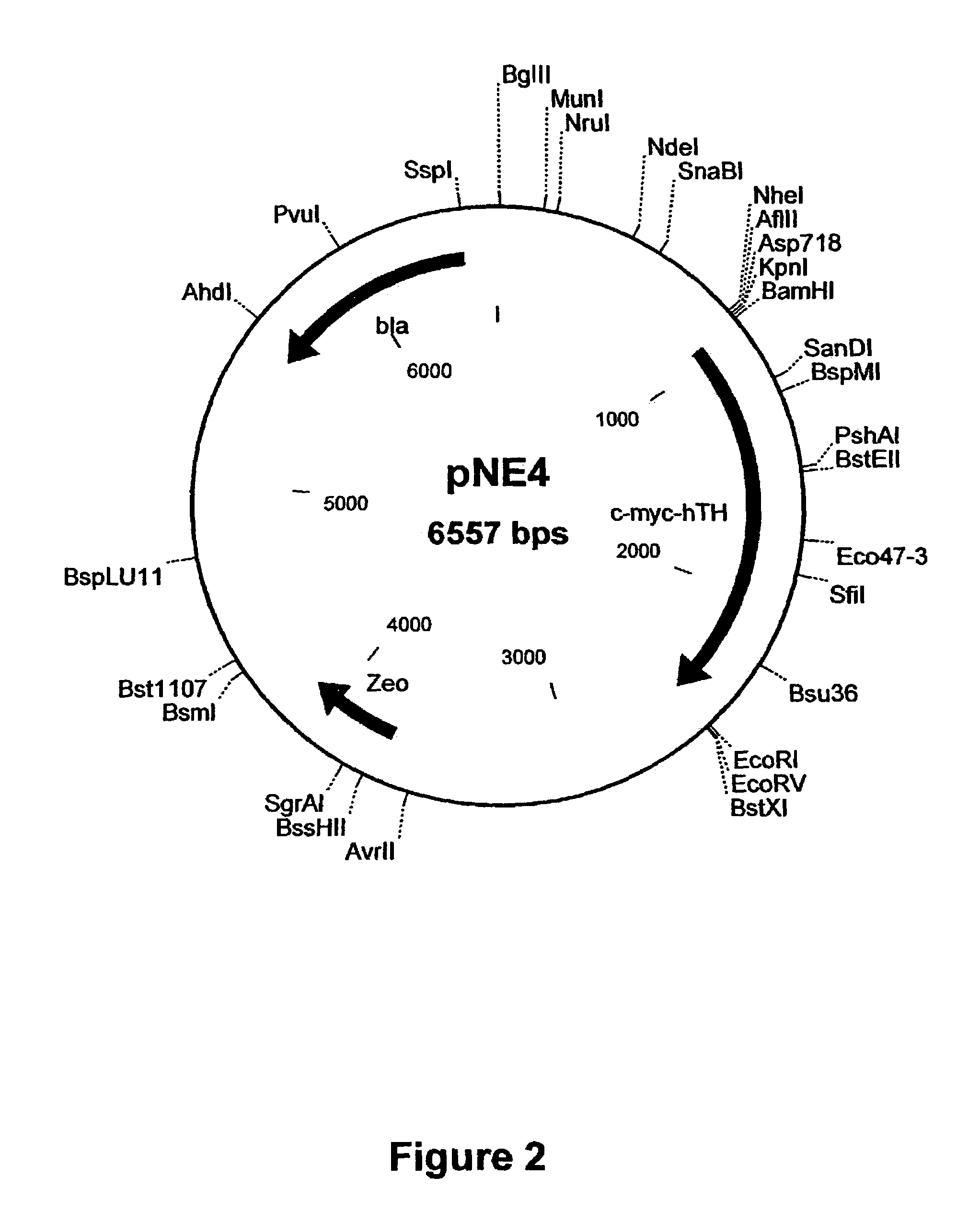
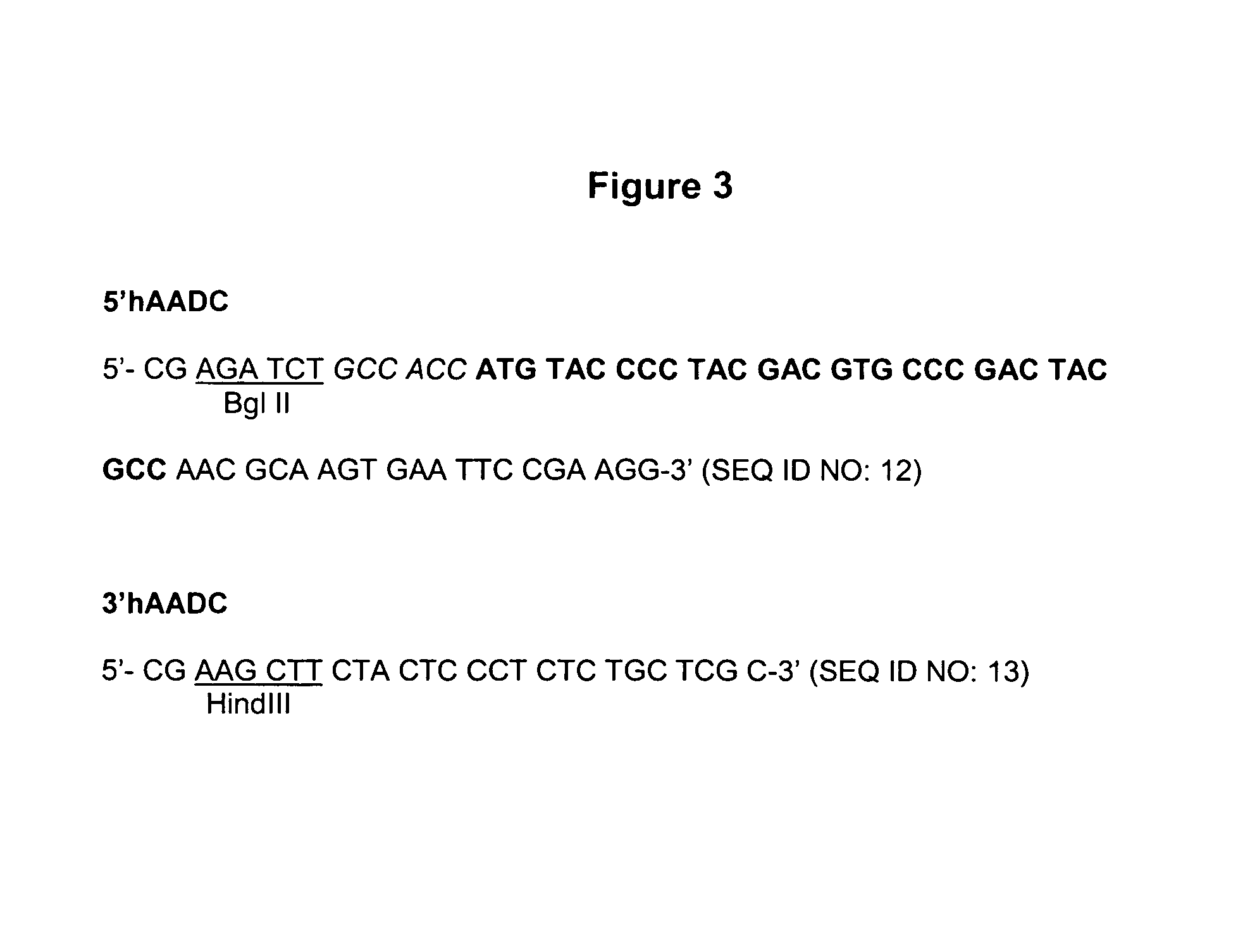
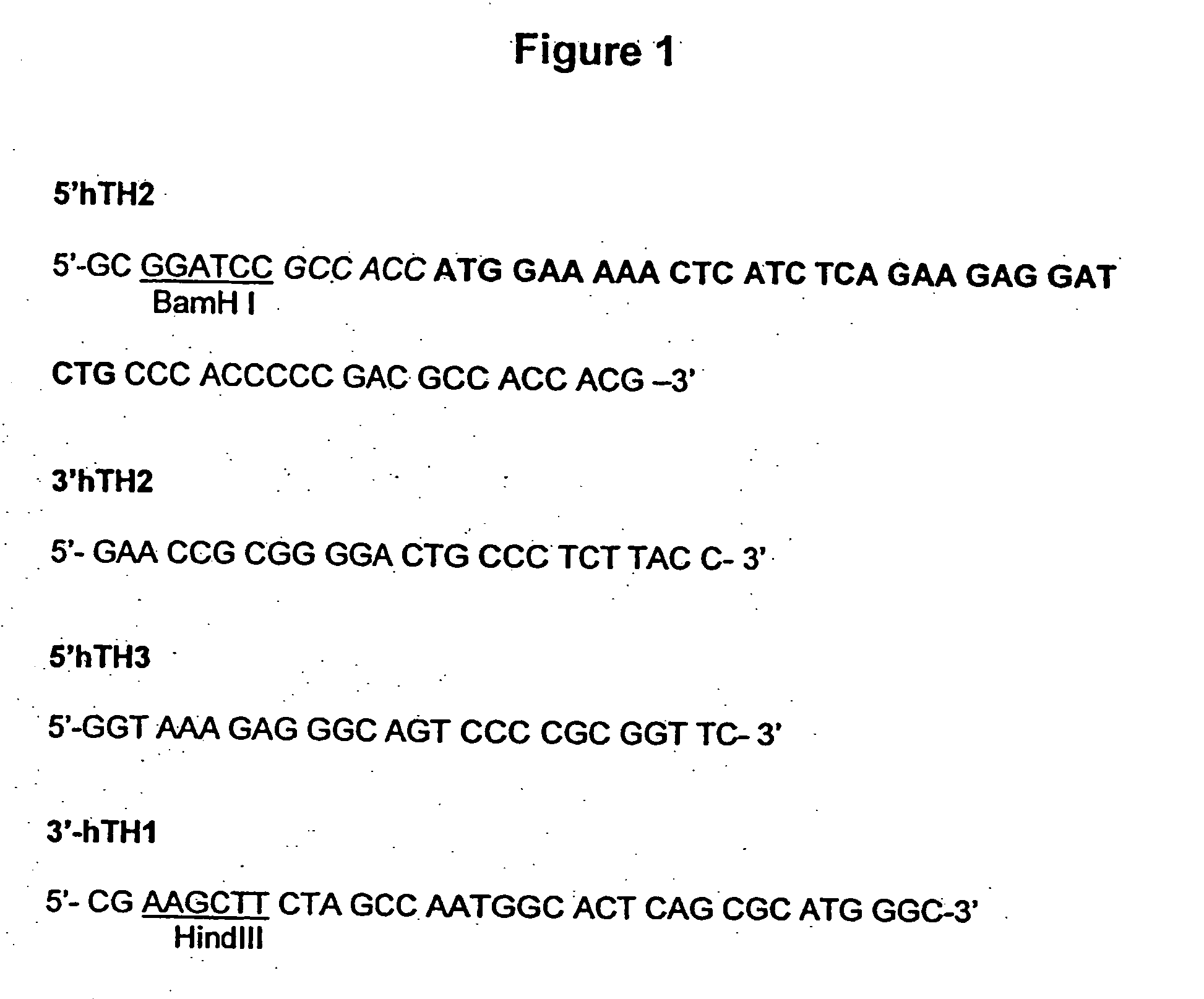
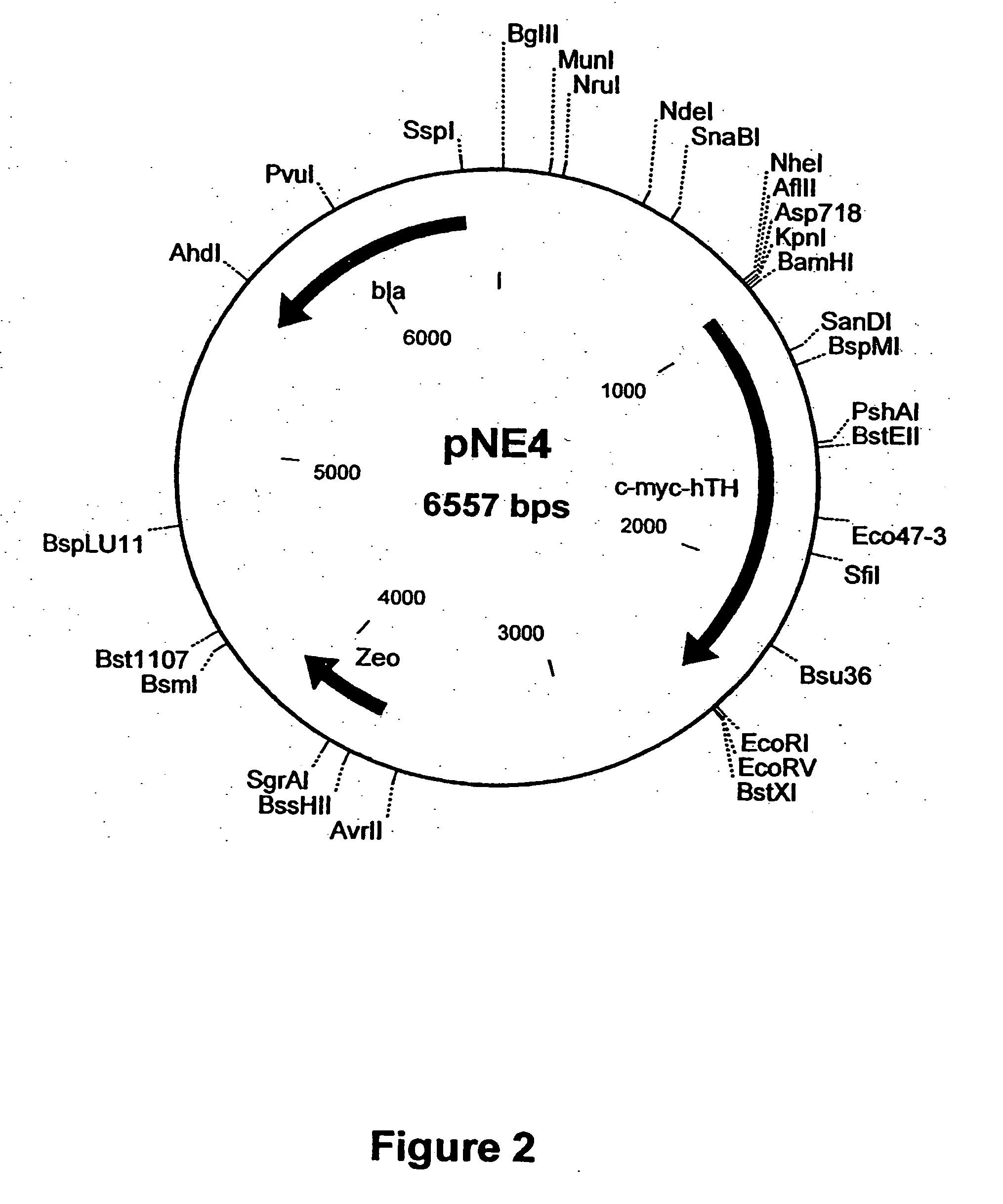
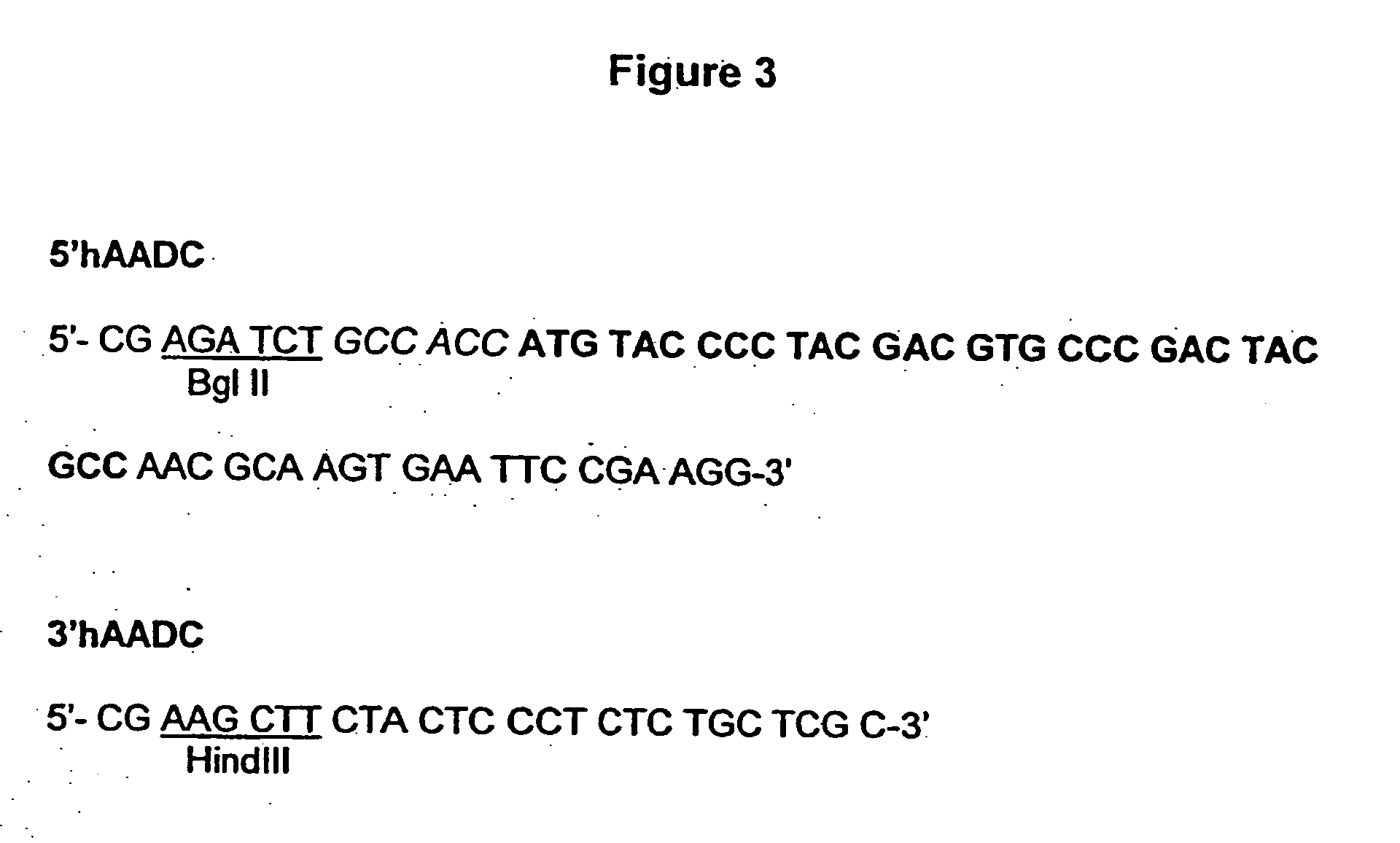
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Vectors for gene trapping and gene activation
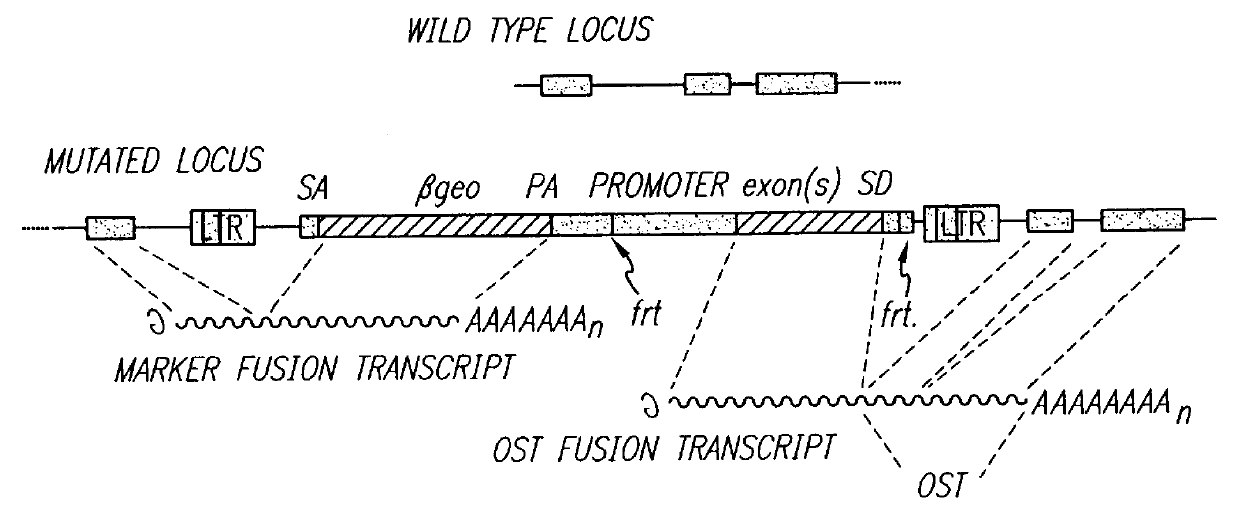
InactiveUS6080576AImprove efficiencySufficient efficiencyNucleic acid vectorMicroorganism librariesAntibiotic resistanceBiological activation
A novel 3' gene trap cassette is described that does not encode a marker conferring antibiotic resistance and can be used to efficiently trap and identify cellular genes. Vectors incorporating the presently 3' gene trap cassette find particular application in gene discovery, the production of transgenic cells and animals, and gene activation.
Owner:LEXICON PHARM INC
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Viral Vectors
InactiveUS20090017543A1Prevent reverse transcriptionAntibody mimetics/scaffoldsGenetic material ingredientsViral vectorVirus
Owner:OXFORD BIOMEDICA (UK) LTD
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
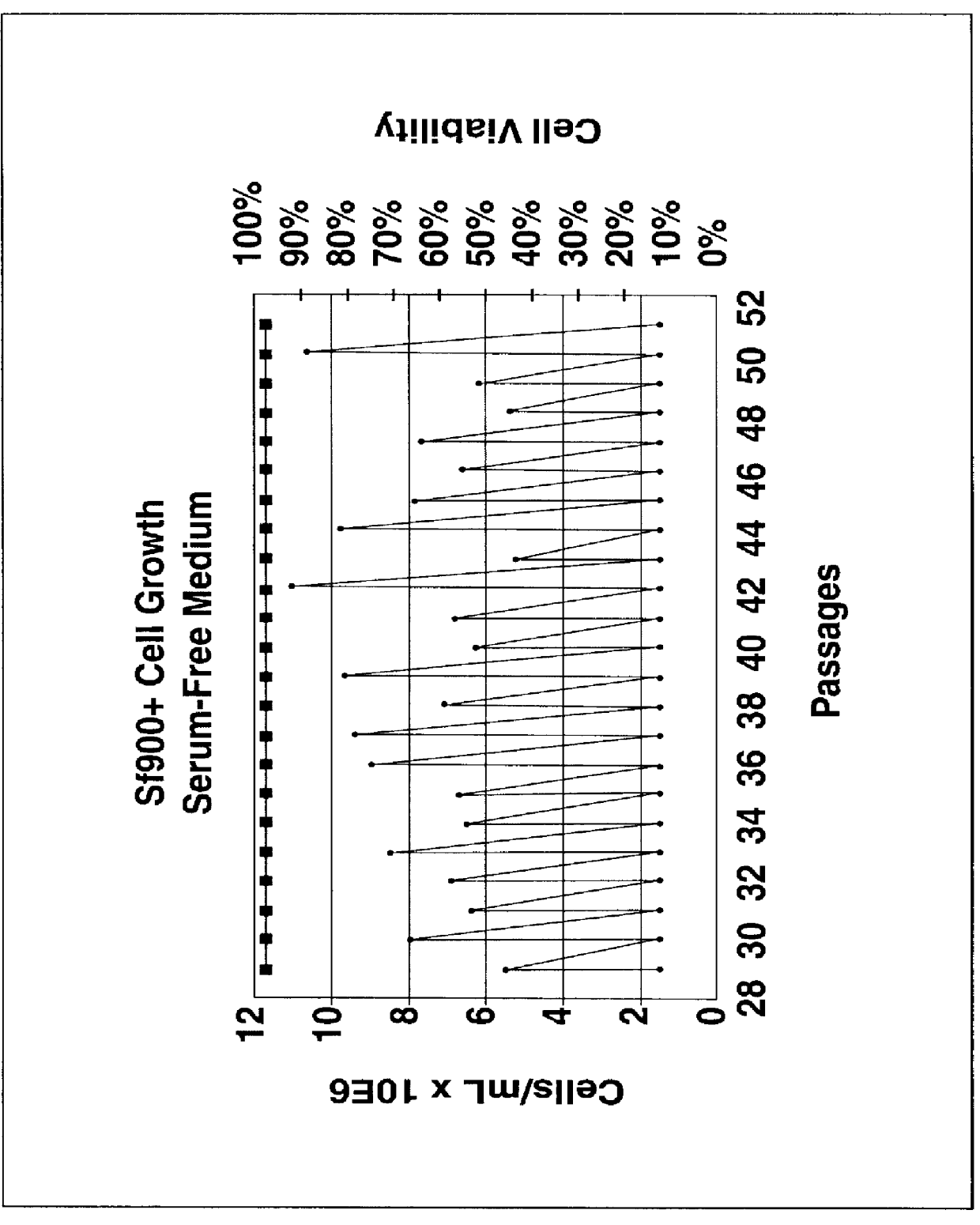
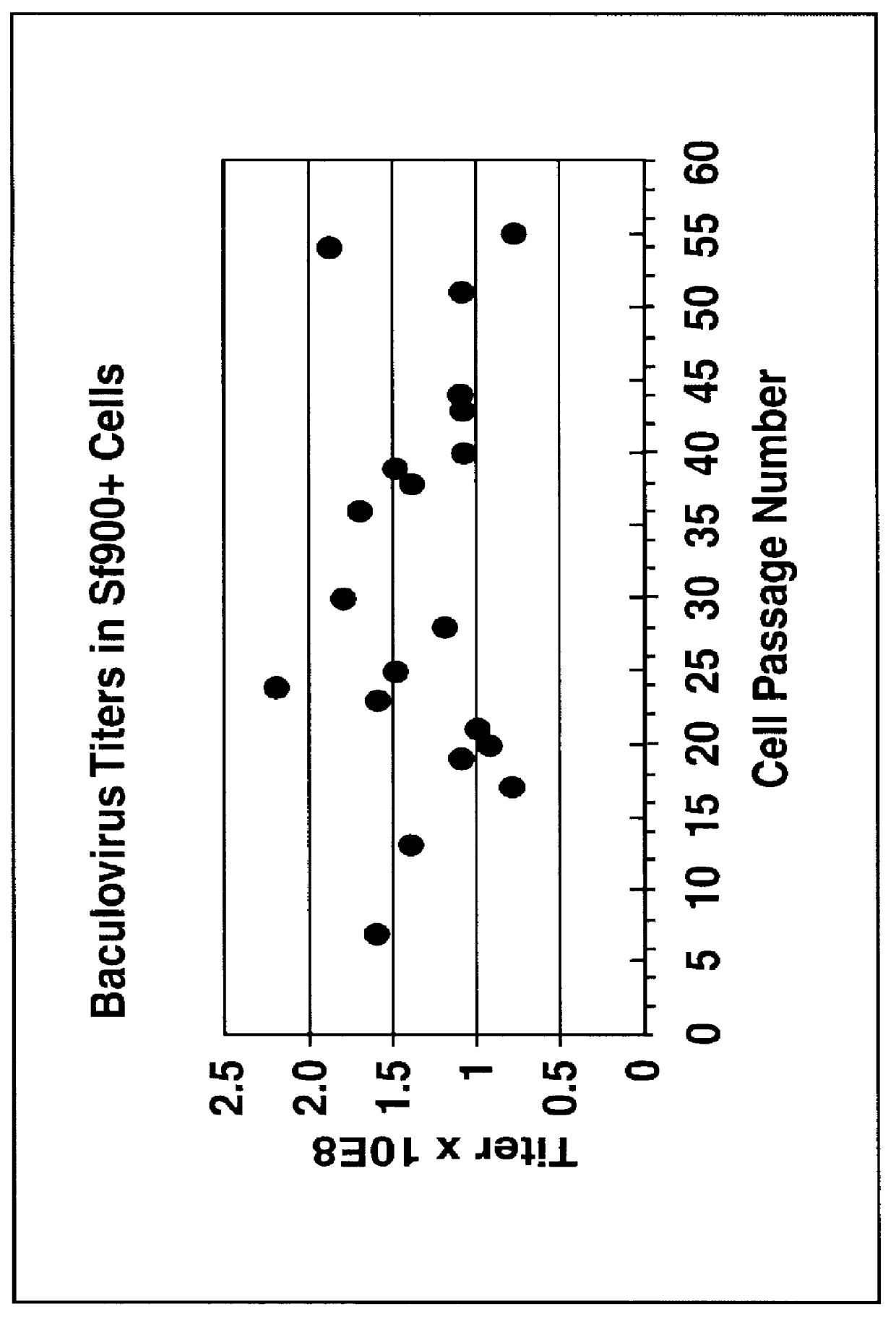
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
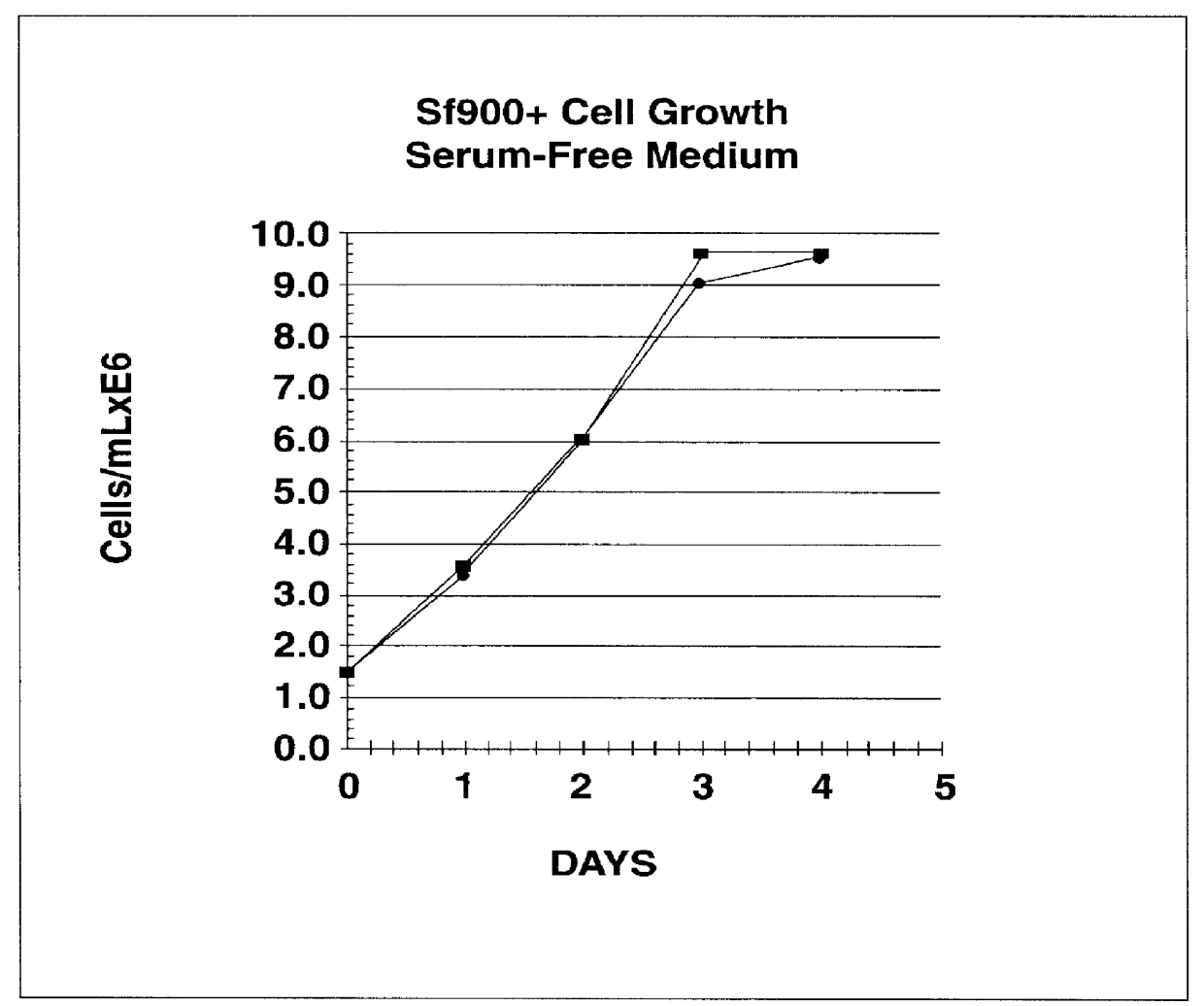
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
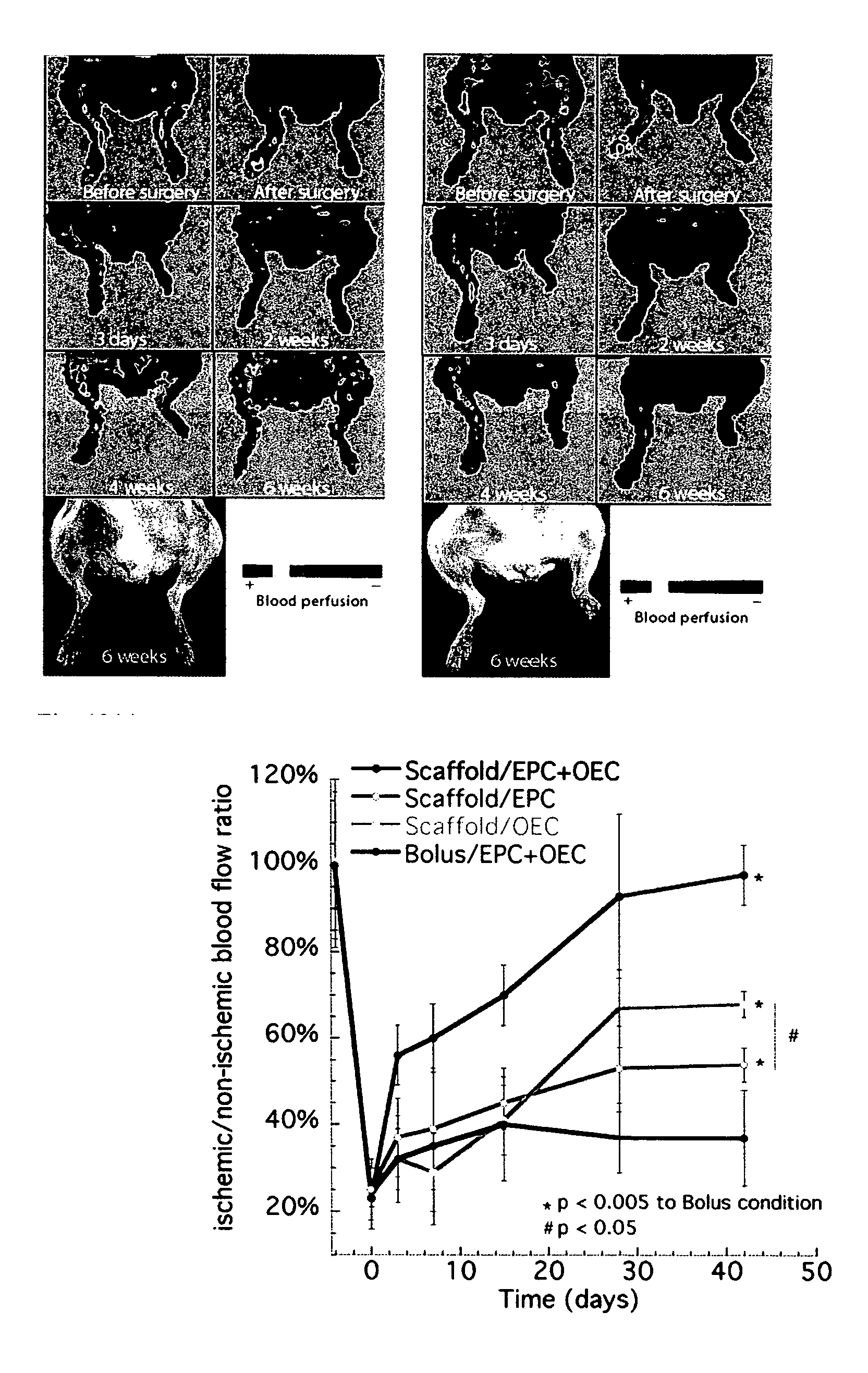
Scaffolds for cell transplantation
ActiveUS20080044900A1Enhance cell viabilityIncrease successBioreactor/fermenter combinationsBiological substance pretreatmentsBiomedical engineeringCell biology
A device that includes a scaffold composition and a bioactive composition with the bioactive composition being incorporated into or coated onto the scaffold composition such that the scaffold composition and / or a bioactive composition controls egress of a resident cell or progeny thereof. The devices mediate active recruitment, modification, and release of host cells from the material.
Owner:RGT UNIV OF MICHIGAN +1
Identifying oligonucleotides for in vitro recombination
“In silico” nucleic acid recombination methods, related integrated systems utilizing genetic operators and libraries made by in silico shuffling methods are provided.
Owner:CODEXIS INC
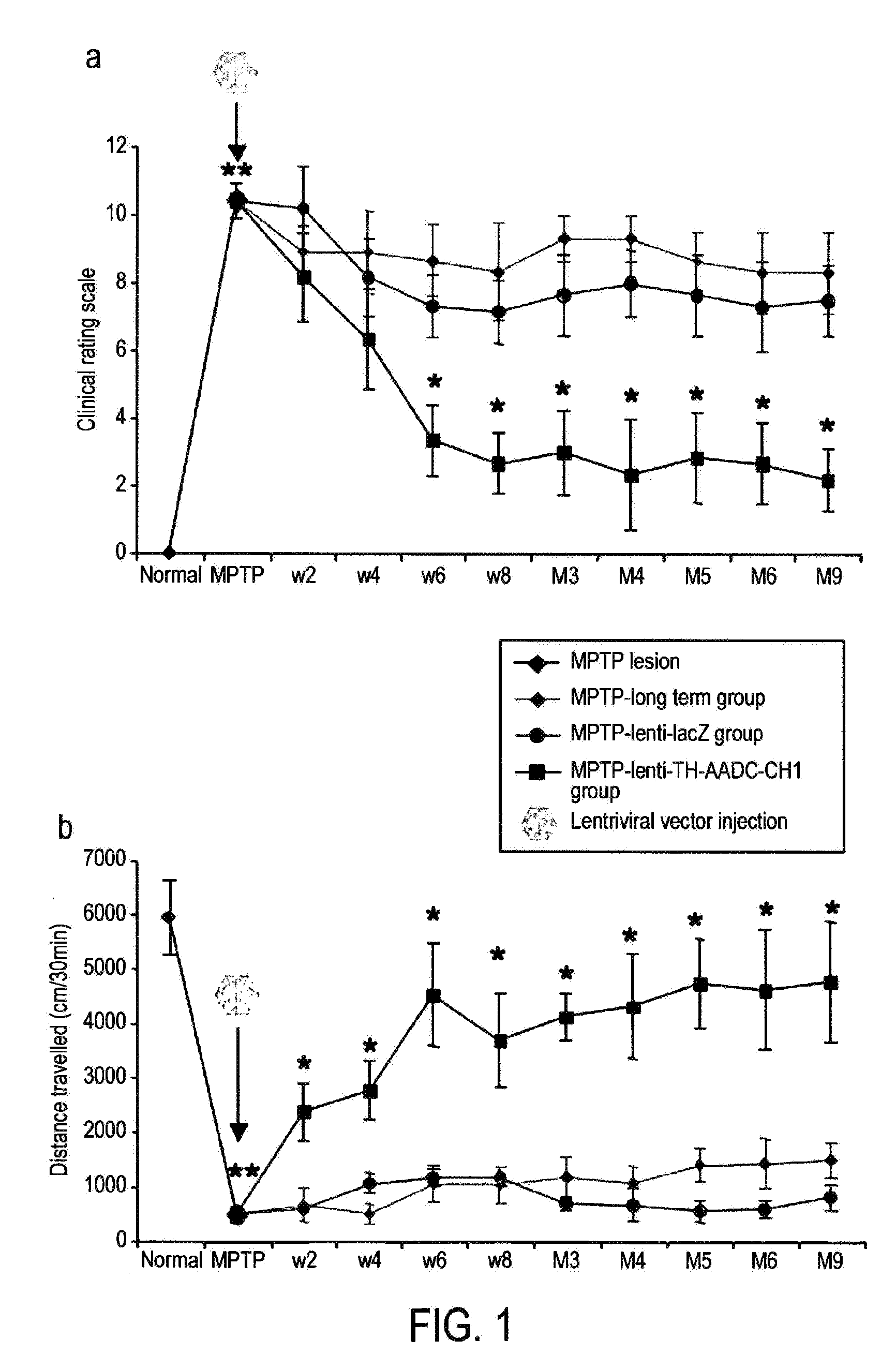
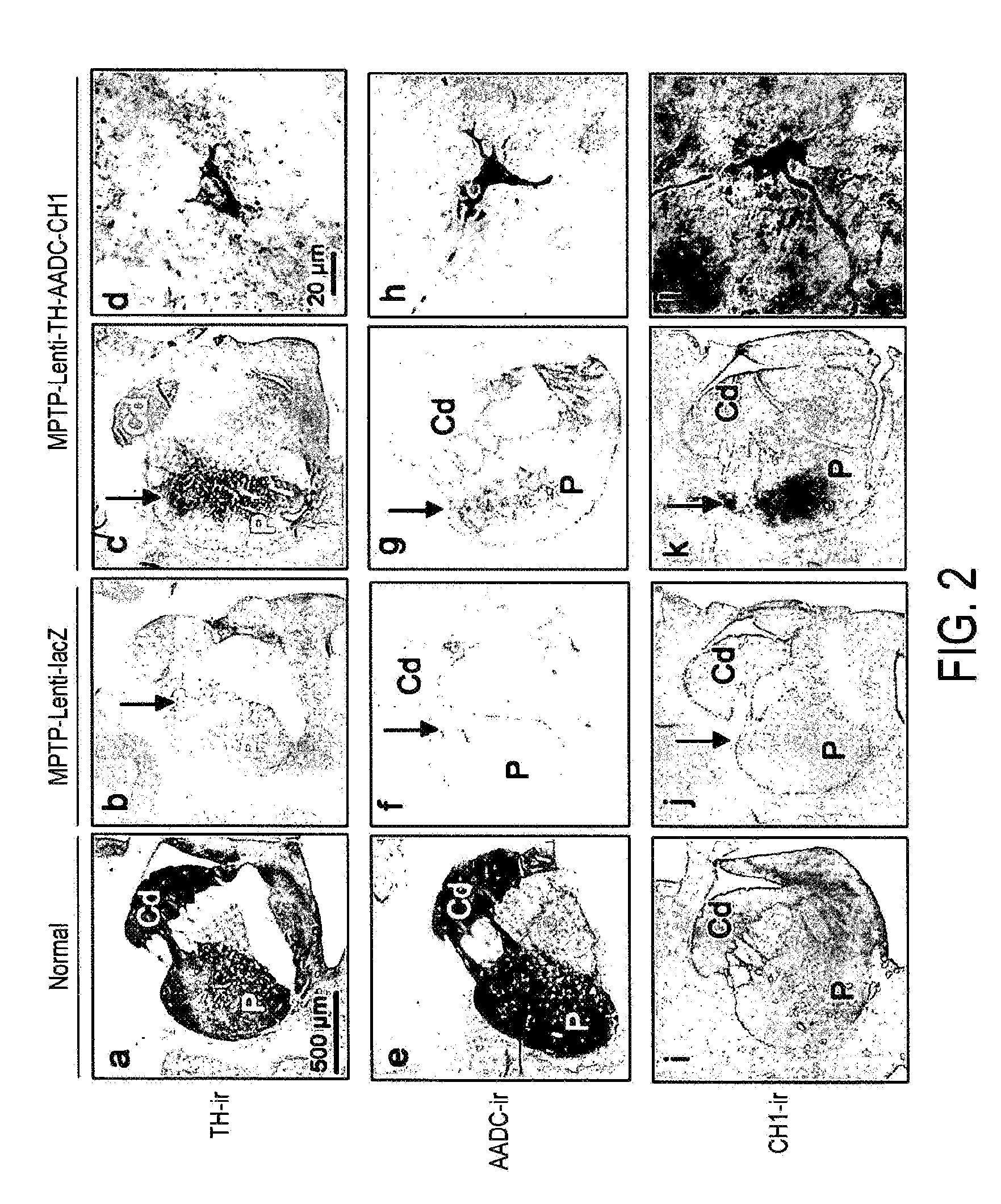
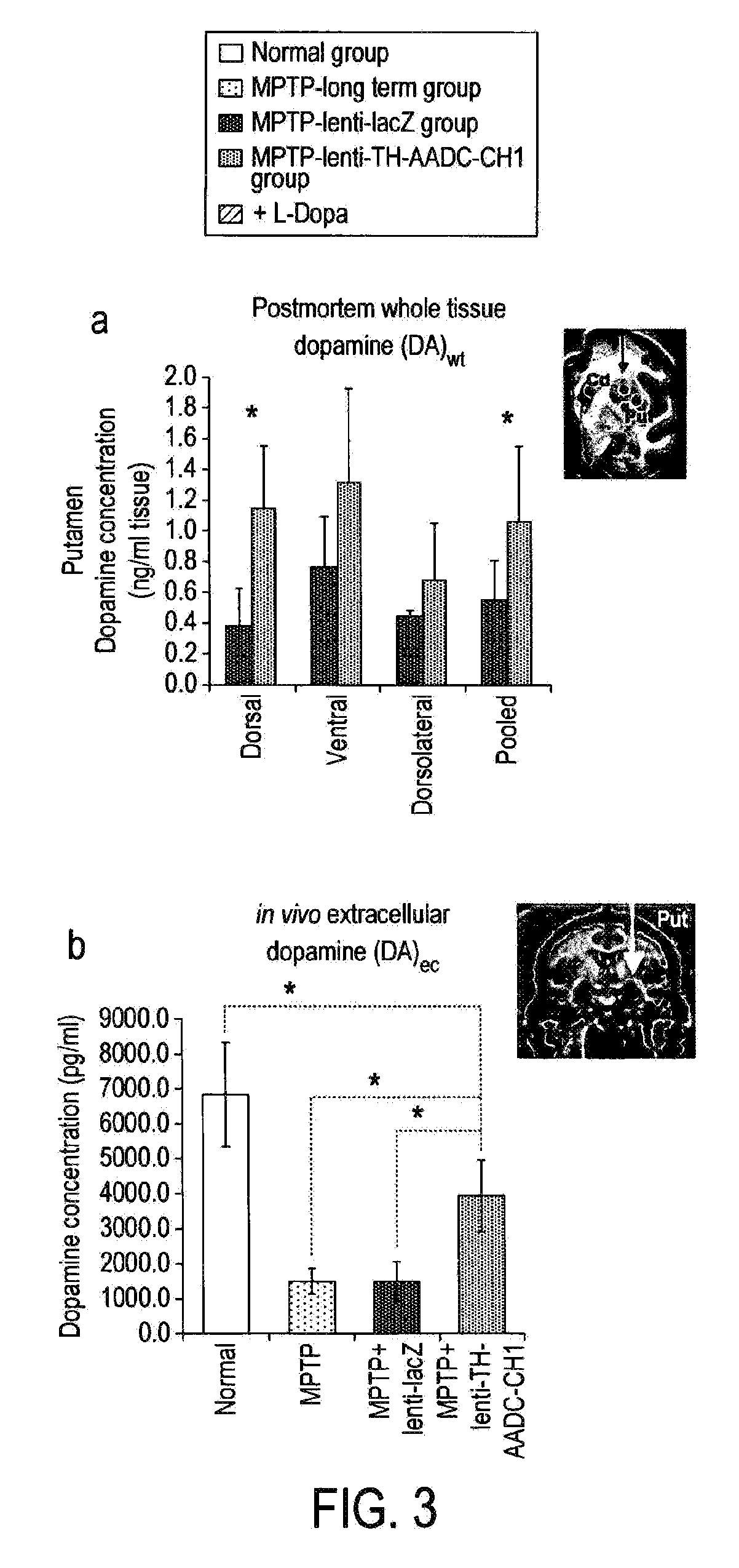
Treatment regimen for parkinson's disease
InactiveUS20120295960A1Reduce potential side effectsReduce maintenanceNervous disorderNucleic acid vectorSide effectCombination therapy
Provided is an improved treatment for Parkinson's Disease where the efficacy of L-Dopa treatment is increased by including gene therapy in the treatment regimen. The combination therapy results in long-term improvements in response to L-Dopa and diminished side effects caused by L-Dopa.
Owner:OXFORD BIOMEDICA (UK) LTD
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
Method for vector delivery
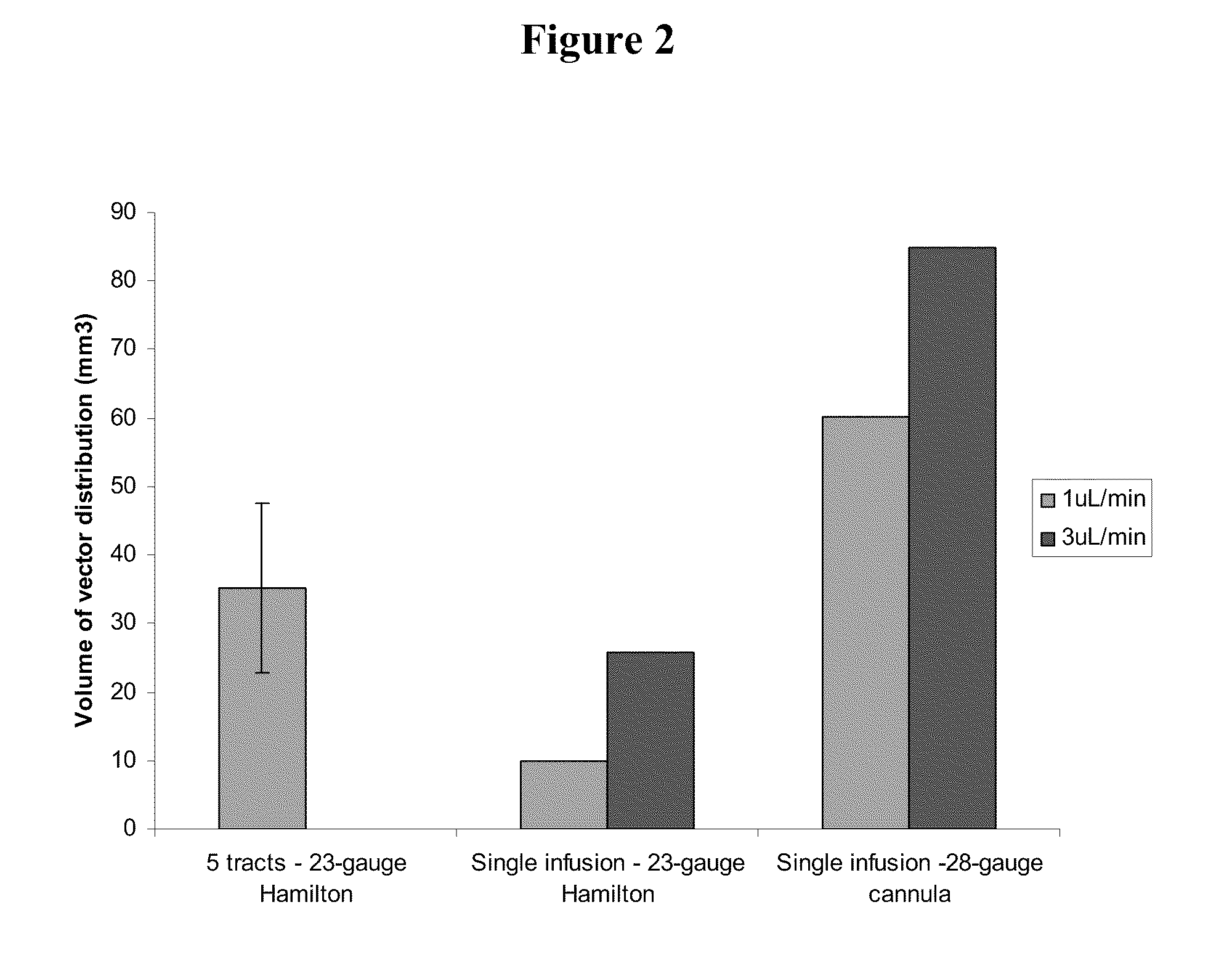
InactiveUS20110293571A1Optimize volumeTotal volume of vector distribution in the brain wasBiocideOrganic active ingredientsNeuroscience
Owner:OXFORD BIOMEDICA (UK) LTD
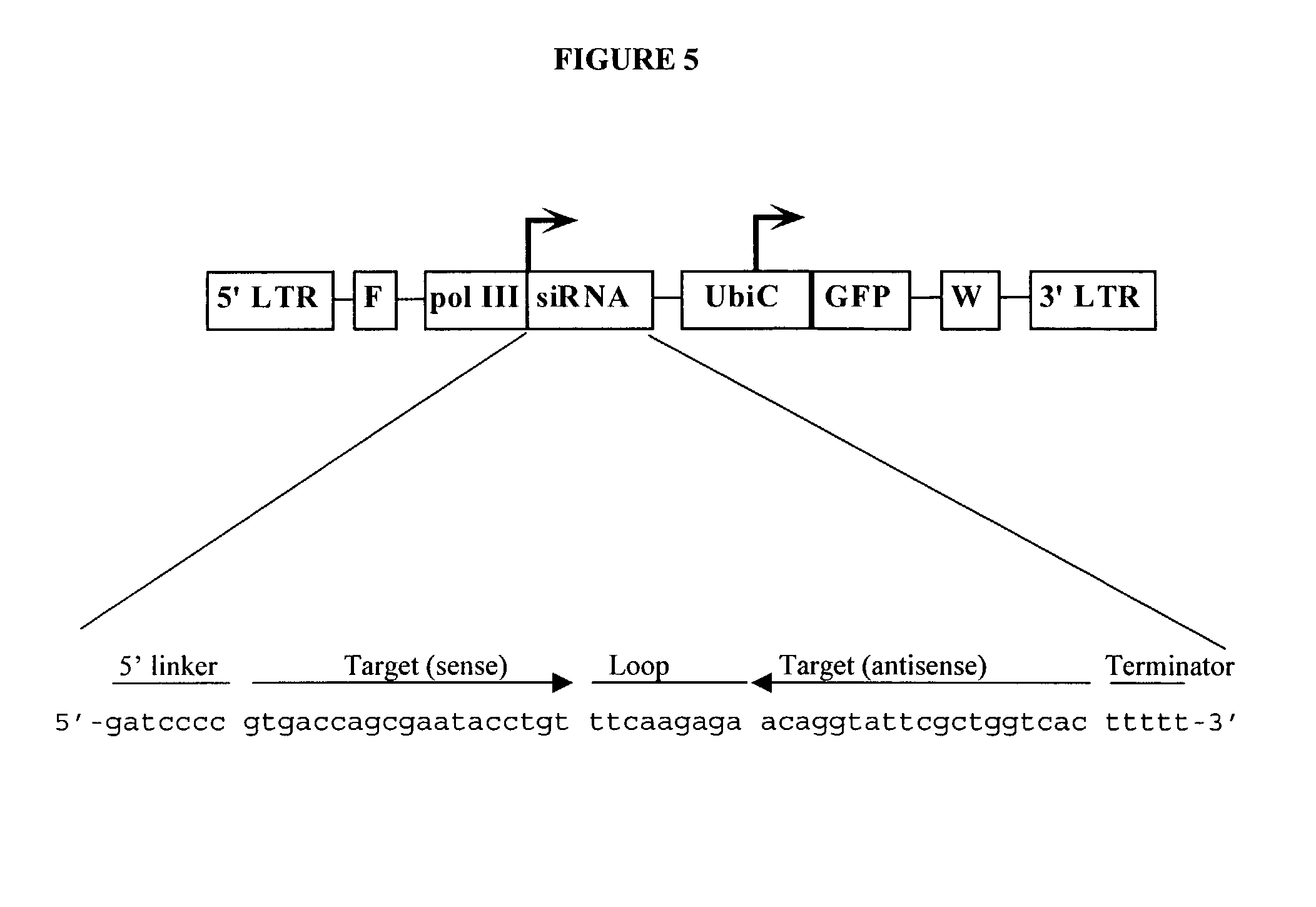
Method for expression of small antiviral RNA molecules within a cell
ActiveUS20030059944A1Prevents sequenceHigh expressionOrganic active ingredientsPeptide/protein ingredientsGeneticsViral life cycle
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV.
Owner:CALIFORNIA INST OF TECH
HIV envelope polypeptides
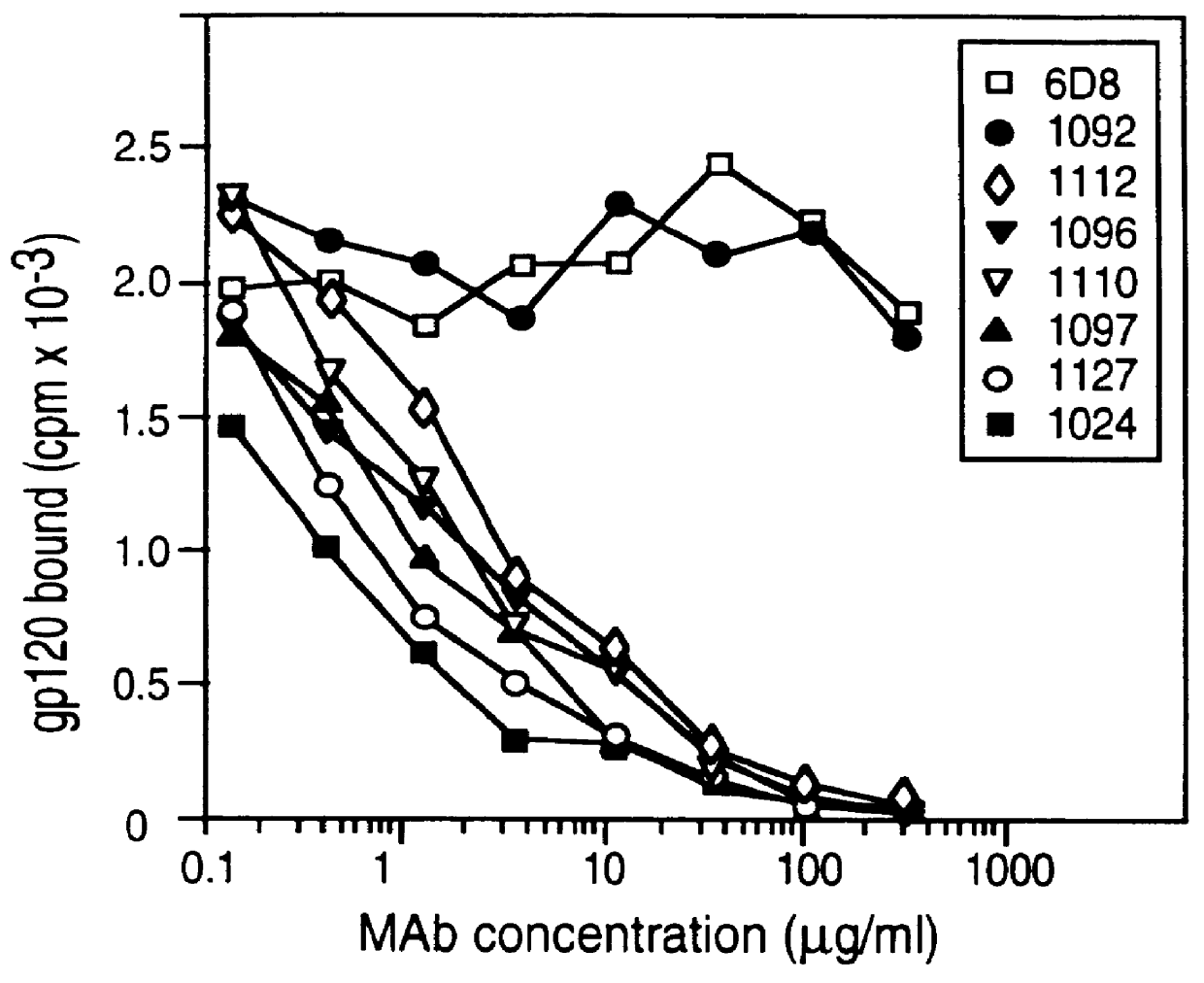
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
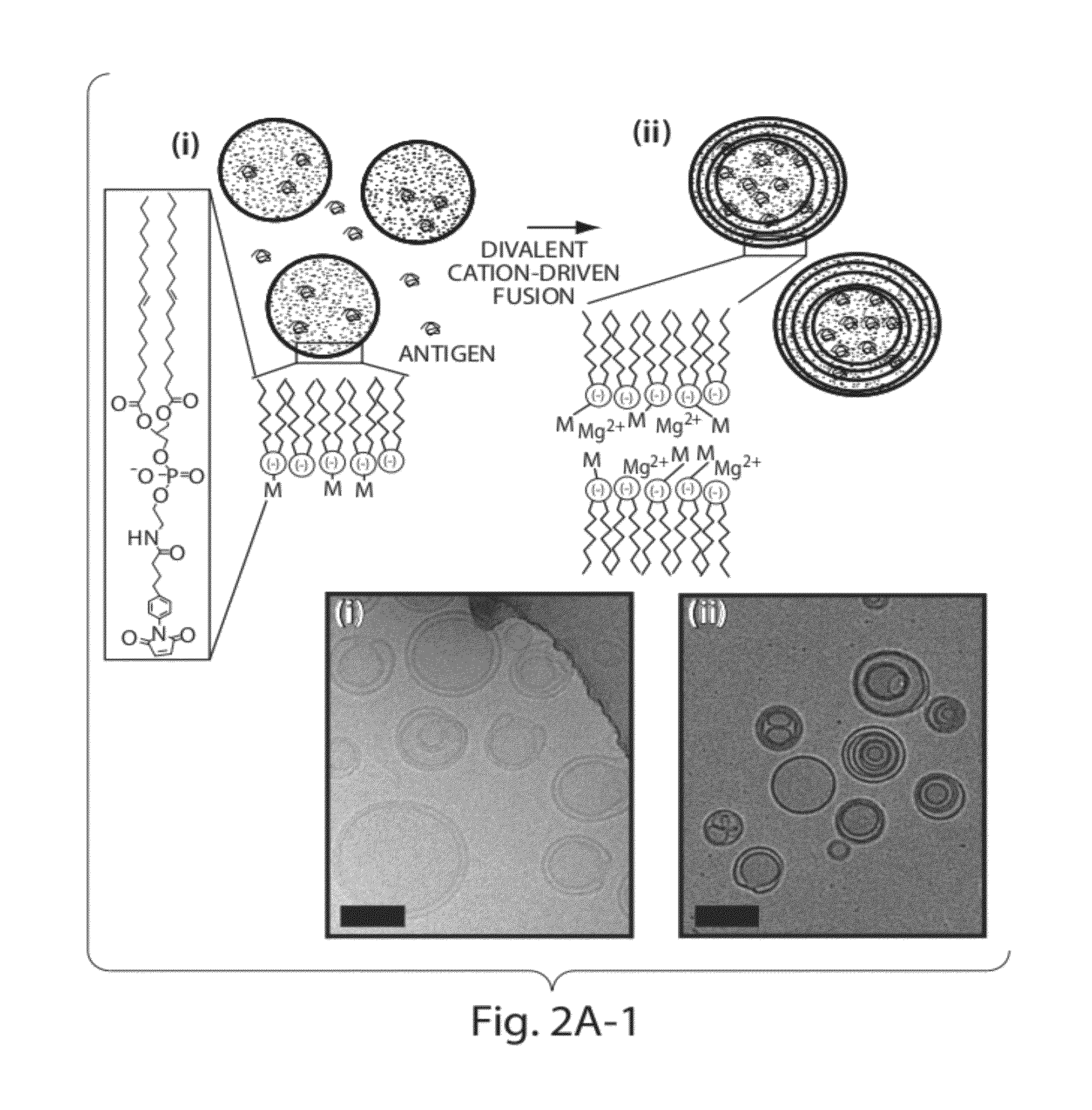
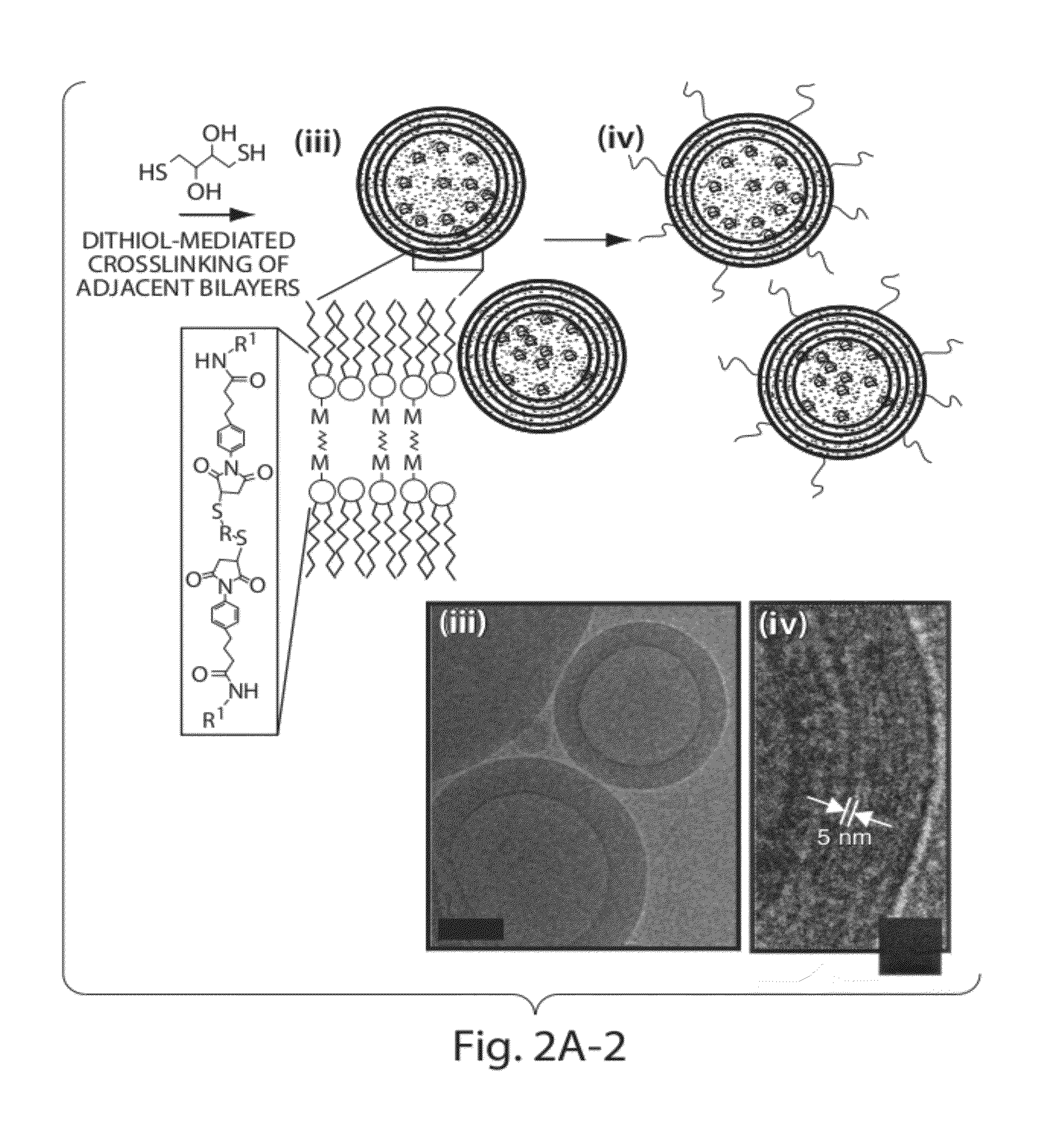
Lipid vesicle compositions and methods of use
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Retroviral vector
InactiveUS7351585B2High titerKeep for a long timeGenetic material ingredientsArtificial cell constructsNucleotideRetrovirus
Provided herein is a retroviral vector comprising, and capable of expressing, a nucleotide of interest (NOI), wherein the NOI encodes an RNA or protein which is harmful to a cell.
Owner:OXFORD BIOMEDICA (UK) LTD
Mammalian viral vectors and their uses
InactiveUS6255071B1Stable episomal maintenanceMaintain relatively stableSugar derivativesMicrobiological testing/measurementRetroviral provirusMammal
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli.In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS20050261229A1Stimulate productionEnhanceVirusesPeptide/protein ingredientsFc receptorAdjuvant
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Yeast-dendritic cell vaccines and uses thereof
Owner:UNIV OF COLORADO THE REGENTS OF +1
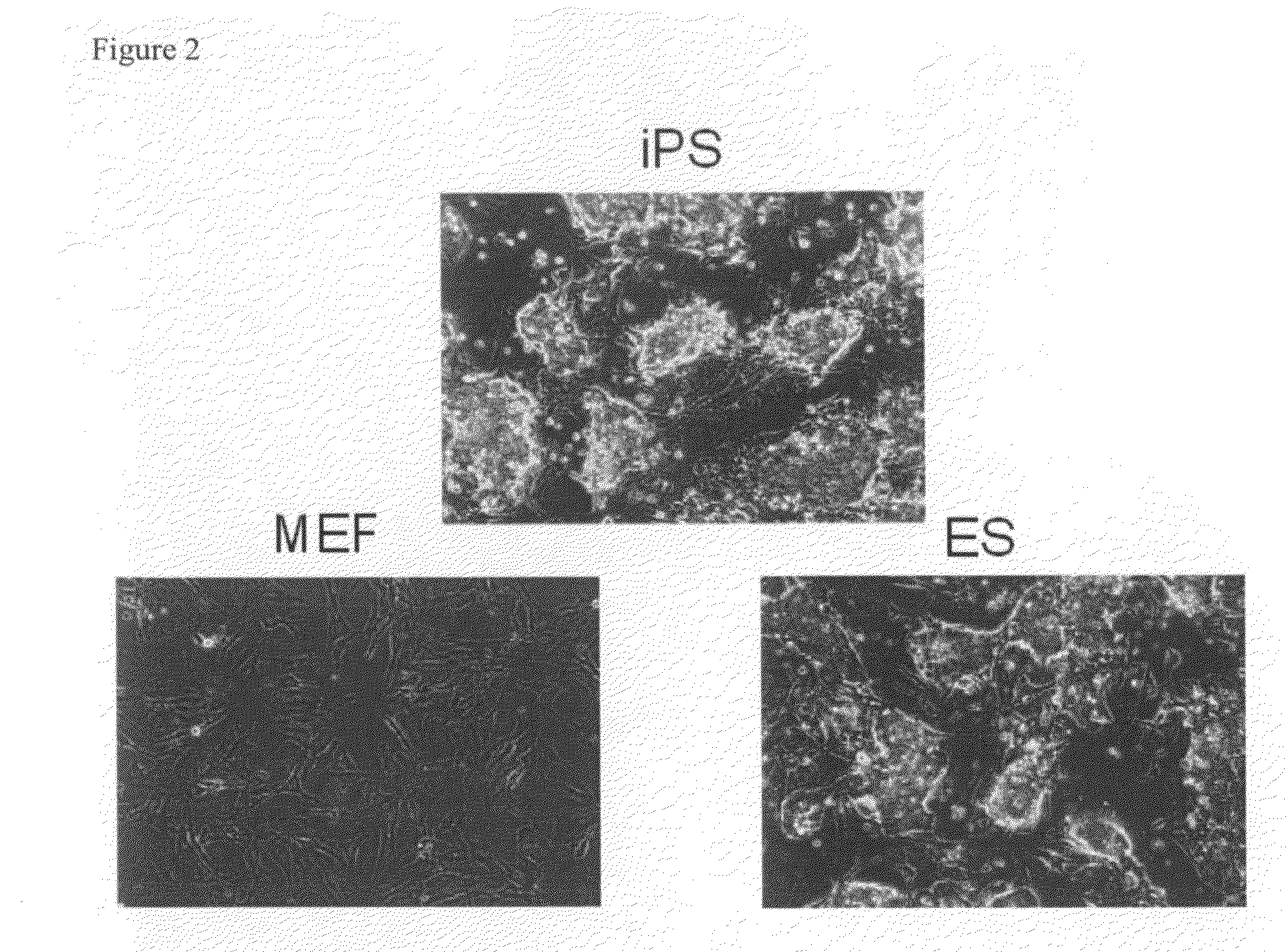
Somatic cell reprogramming by retroviral vectors encoding Oct3/4. Klf4, c-Myc and Sox2
ActiveUS8129187B2Easy to prepareEffective isolationGenetically modified cellsArtificial cell constructsNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
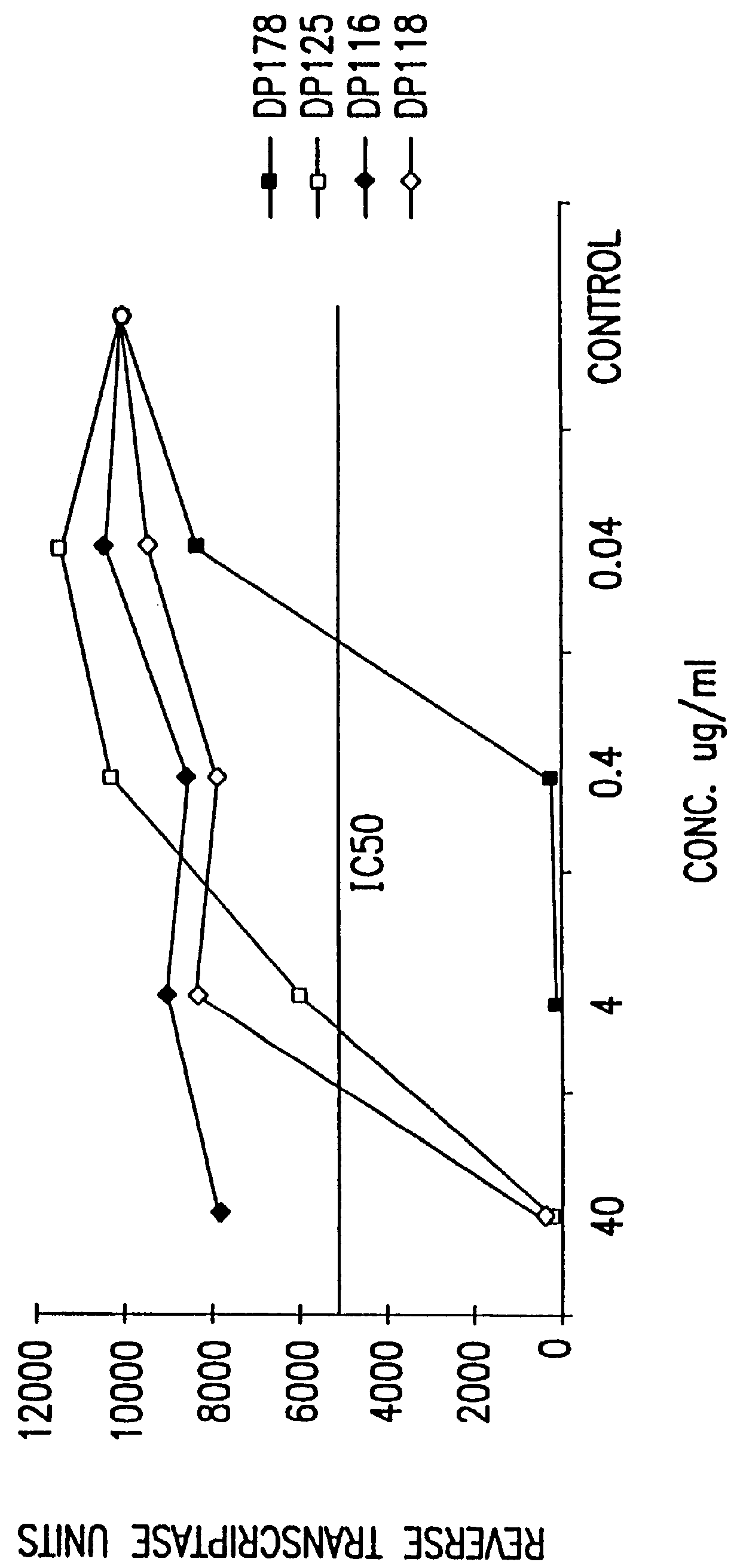
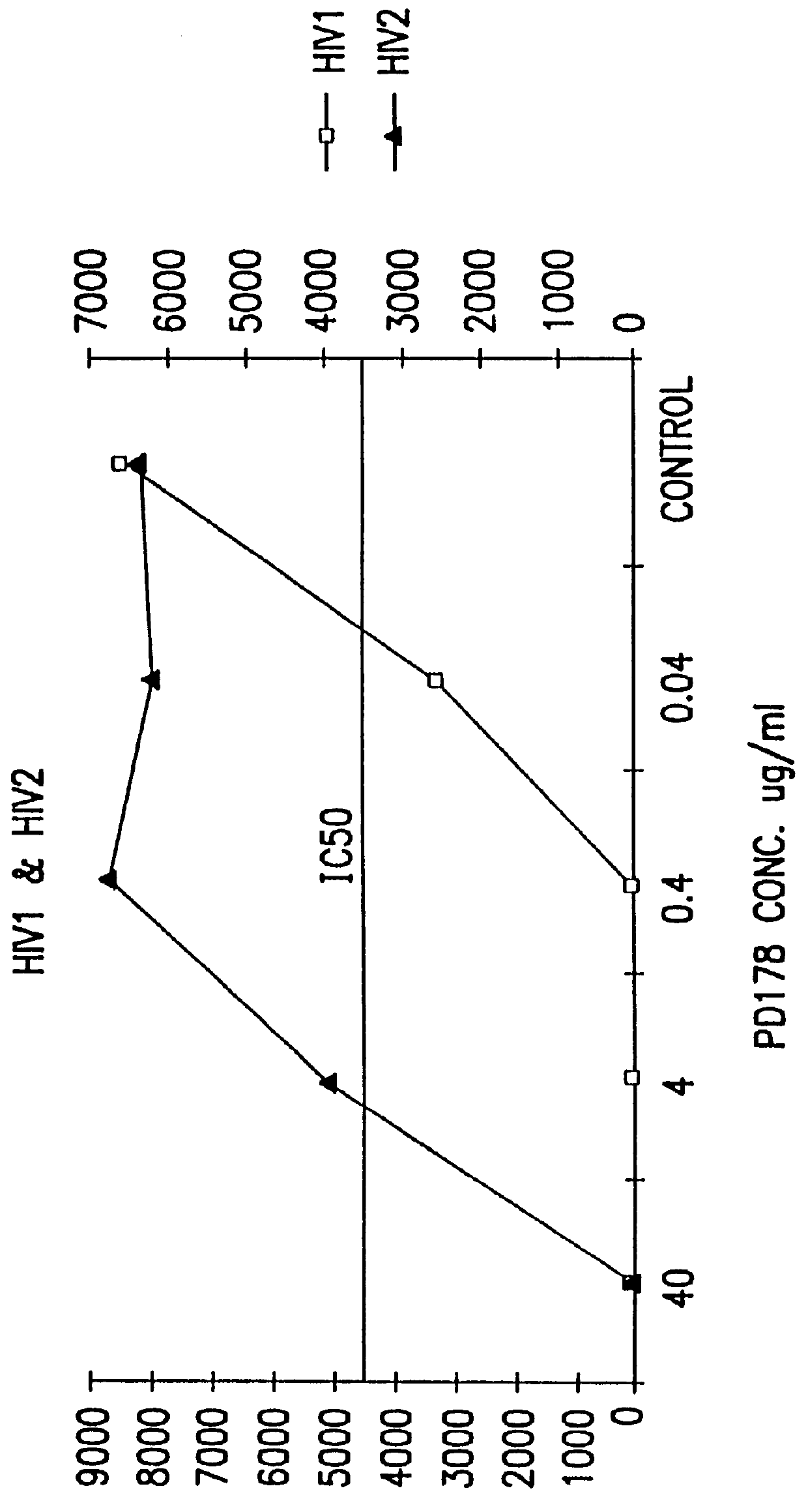
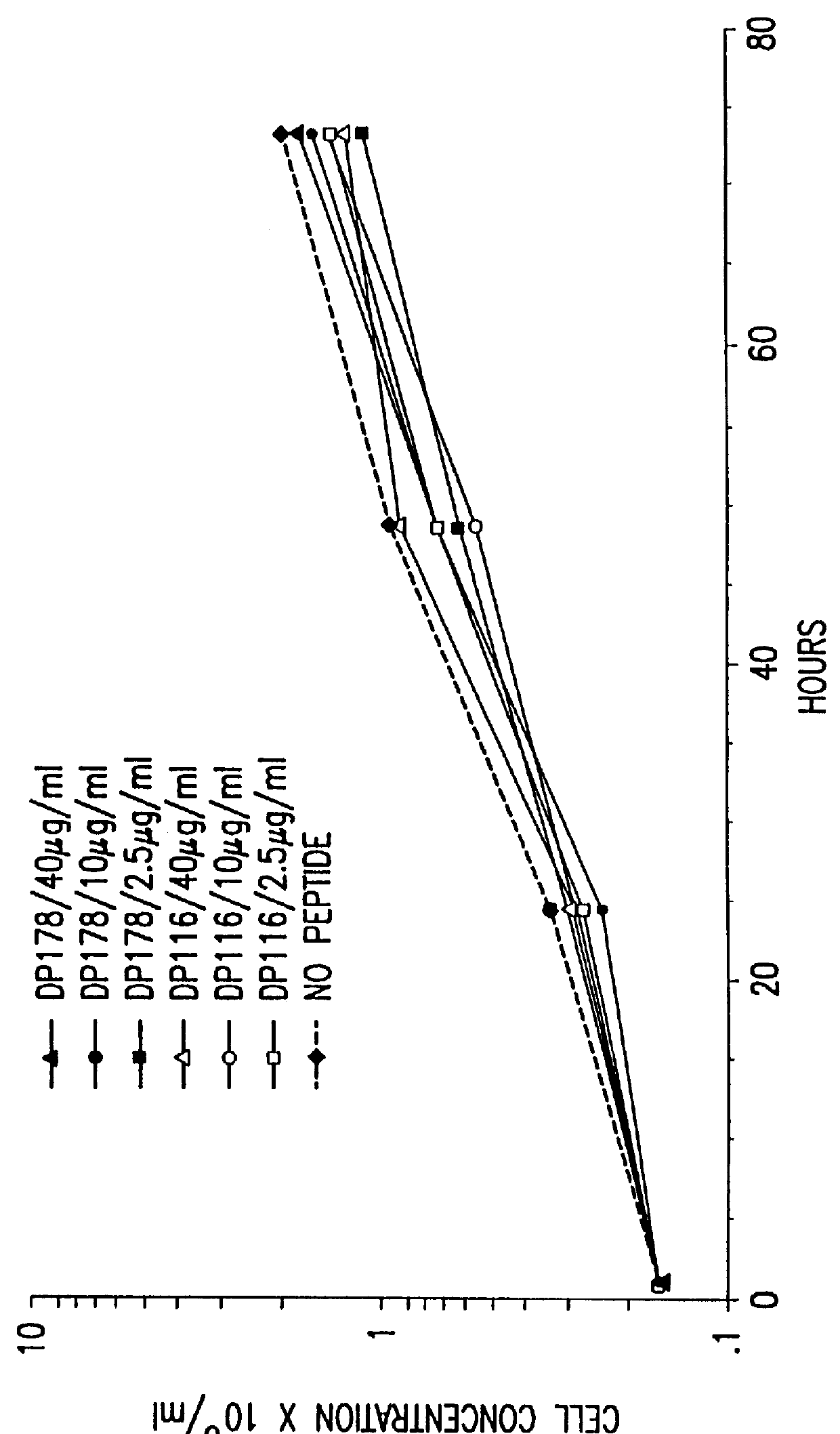
Simian immunodeficiency virus peptides with antifusogenic and antiviral activities
InactiveUS6017536ASsRNA viruses negative-sensePeptide/protein ingredientsSimian immunodeficiency viruses SIVDefective virus
The present invention relates to peptides which exhibit antifusogenic and antiviral activities. The peptides of the invention consist of a 16 to 39 amino acid region of a simian immunodeficiency virus (SIV) protein. These regions were identified through computer algorithms capable of recognizing the ALLMOTI5, 107x178x4, or PLZIP amino acid motifs. These motifs are associated with the antifusogenic and antiviral activities of the claimed peptides.
Owner:TRIMERIS +1
Vectors
InactiveUS20060281180A1Stable long-term expressionEfficient expressionAntibacterial agentsFactor VIIGeneticsViral vector
Provided is a lentiviral vector capable of delivering a nucleotide of interest (NOI) to a desired target site and wherein the NOI encodes the Factor VIII and the Factor VIII is expressed following delivery of the NOI to the desired target site.
Owner:OXFORD BIOMEDICA (UK) LTD
Transgenic organism
InactiveUS20090007284A1Efficient productionEfficiency advantageAnimal cellsVectorsNucleotide sequencingPolynucleotide
A method of producing a transgenic cell comprising introducing into a cell a non-primate lentiviral expression vector comprising a nucleotide of interest (NOI). Also described is a method of producing a transgenic cell comprising introducing into a cell a lentiviral expression vector comprising a NOI capable of generating an antisense oligonucleotide, a ribozyme, an siRNA, a short hairpin RNA, a micro-RNA or a group 1 intron. Also described is a viral vector comprising a first nucleotide sequence, wherein said first nucleotide sequence comprises: (a) a second nucleotide sequence comprising an aptazyme; and (b) a third nucleotide sequence capable of generating a polynucleotide; wherein (a) and (b) are operably linked and wherein the aptazyme is activatable to cleave a transcript of the first nucleotide sequence such that said polynucleotide is generated.
Owner:RADCLIFFE PHILIPPA +2
Popular searches
Dead animal preservation Immunoglobulins against cell receptors/antigens/surface-determinants Mammal material medical ingredients Blood/immune system cells Animal repellants Bacteriophages Bacterial antigen ingredients Cell receptors/surface-antigens/surface-determinants Antibody ingredients Cell culture active agents
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com