A novel oncolytic virus and its preparation method and application
An oncolytic virus, a new type of technology, applied in the field of biomedicine, can solve problems such as weakening inhibitory activity, and achieve the effects of improving killing function, good targeting and anti-tumor effect, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction and expression verification of recombinant vaccinia virus rTV-mIL-21 of mouse IL-21
[0033] 1.1 Construction of pSC65 vector with mouse IL-21 gene of interest
[0034] The DNA sequence of mouse-derived IL-21 was artificially synthesized. The synthetic sequence is shown in SEQ ID NO: 1, and its amino acid sequence is shown in SEQ ID NO: 2. Using the synthetic DNA sequence as a template, the following primers were used for PCR amplification .
[0035] The primers for amplification are:
[0036] Mouse-derived IL-21-F: GTACCAGGCCTAGTACTATGGAGAGGACCCTTGTCTG
[0037] Mouse-derived IL-21-R: AATAAGCTCGAAGTCGAC CTAGGAGAGATGCTGATG
[0038] PCR reaction program: pre-denaturation at 94°C for 5 minutes; denaturation at 98°C for 10 seconds, 58°C: annealing for 30 seconds, extension at 72°C for 1 minute, and 30 cycles of reaction; 72°C loop extension for another 10 minutes, and terminated at 25°C.
[0039] Recovery of PCR products and clone construction:...
Embodiment 2
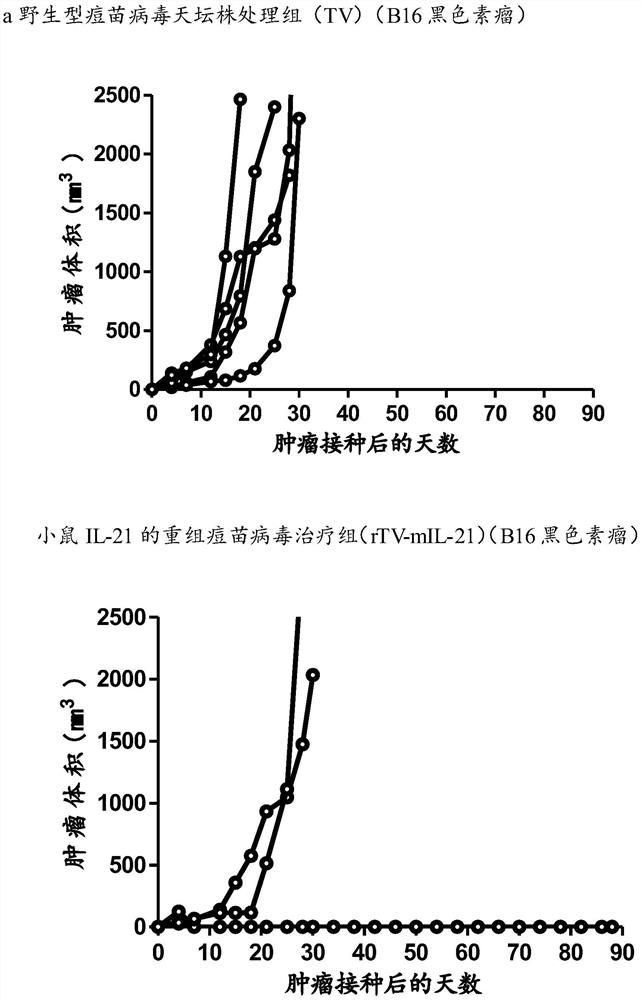
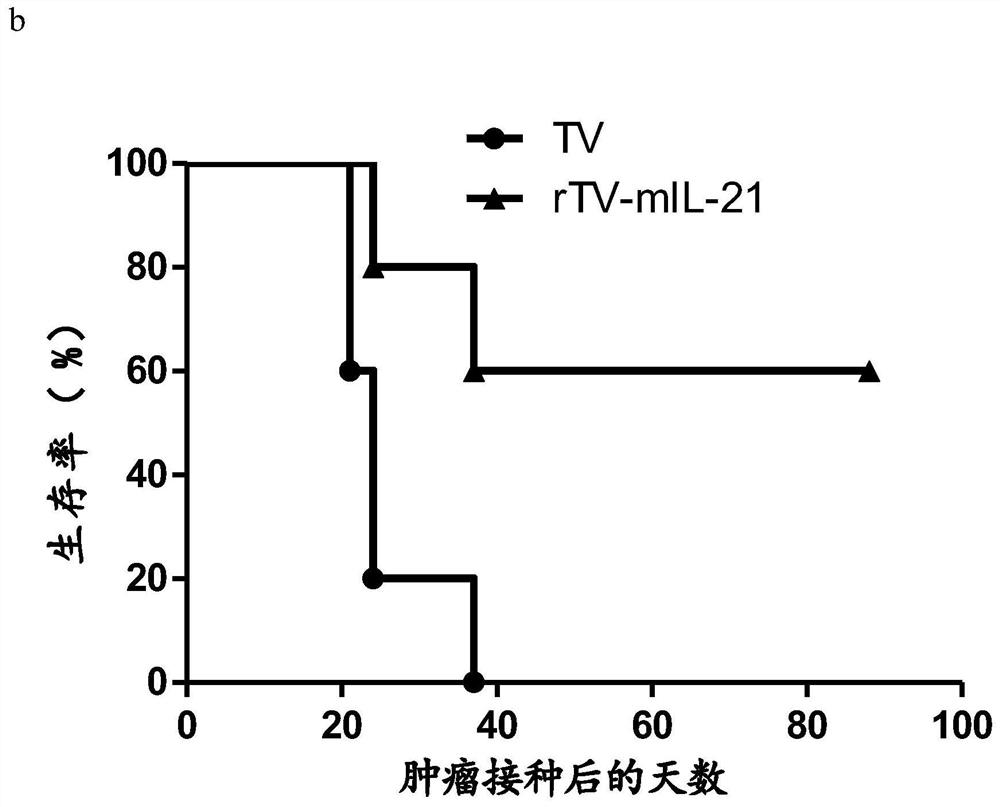
[0058] Example 2: Anti-melanoma effect of mouse IL-21 recombinant vaccinia virus
[0059] 2.1 Amplification and purification of mouse IL-21 recombinant vaccinia virus
[0060] 1. VERO cell plating: 10 cm dish, each dish about 5×10 6 It is advisable to ensure that the cell density reaches 100% when the vaccinia virus is inoculated the next day;
[0061] 2. Before virus inoculation, the complete medium needs to be replaced with 8 mL of maintenance medium (DMEM medium + 2% FBS + 1% PS), and the virus is inoculated into the cells in the maintenance medium at an MOI of about 0.02 (MOI= virus PFU / cell number). Continue to incubate in an incubator at 37°C and 5% CO2 for about 48 hours, and collect samples according to the formation of virus plaques;
[0062] 3. To collect vaccinia virus: Discard 8 mL of medium in the dish, take 2 mL of maintenance medium to blow off the remaining cells, and collect in a 15 mL centrifuge tube;
[0063] 4. After freezing for 24 hours, freeze ...
Embodiment 3
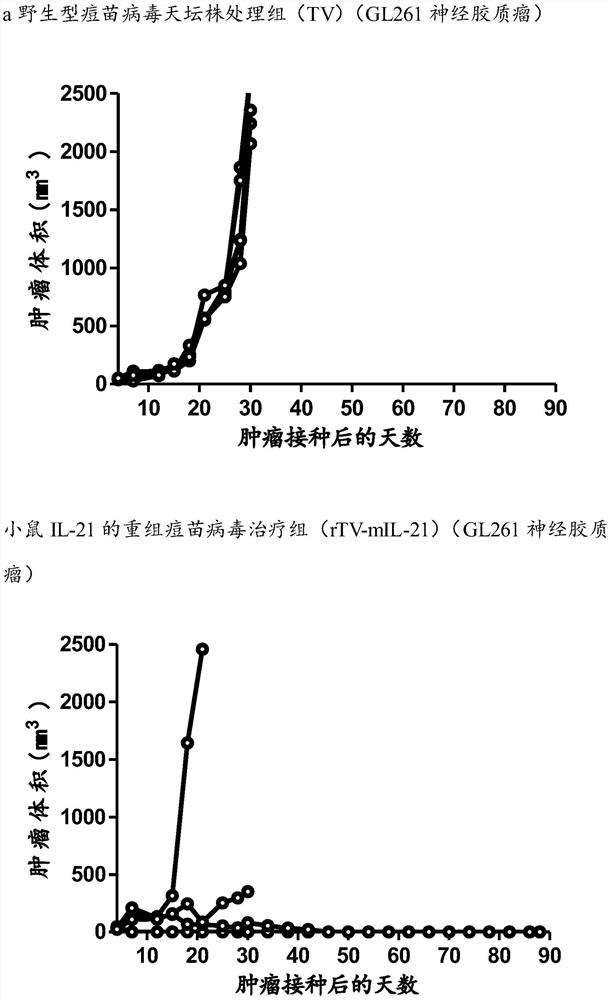
[0080] Example 3: In vivo anti-glioma effect of mouse IL-21 recombinant vaccinia virus
[0081] 1. For C57BL / 6 mice by subcutaneous implantation 1×10 6 Glioma GL261 cells (125 x 25), the long and short diameters of the tumors of the mice were recorded every day, and the volume of the tumor was calculated using the following formula.
[0082] 2. Tumor volume calculation formula: tumor volume (mm 3 ) = (long diameter × wide diameter 2 ) / 2.
[0083] 3. Seven days after the mice were inoculated with glioma GL261 cells, the tumor-forming mice were randomly divided into three groups (5 mice in each group), which were the control group and the wild-type vaccinia virus Tiantan strain (TV) treatment group Recombinant vaccinia virus (rTV-mIL-21) treatment group with mouse IL-21. The administration method of the oncolytic virus is intratumoral injection and reinfusion, and it is obtained from the purified oncolytic virus in Example 2, and is administered once.
[0084] A: Control gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com