Pitavastatin calcium double-layer osmotic pump controlled-release tablet and preparation method thereof
A controlled-release technology of pitavastatin calcium and osmotic pumps, which is applied in pharmaceutical formulations, drug delivery, pill delivery, etc., can solve the problem of drug release stability, poor drug safety, reduced drug release speed, and drug inability to release To avoid large fluctuations in blood drug concentration, reduce individual differences, and reduce the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
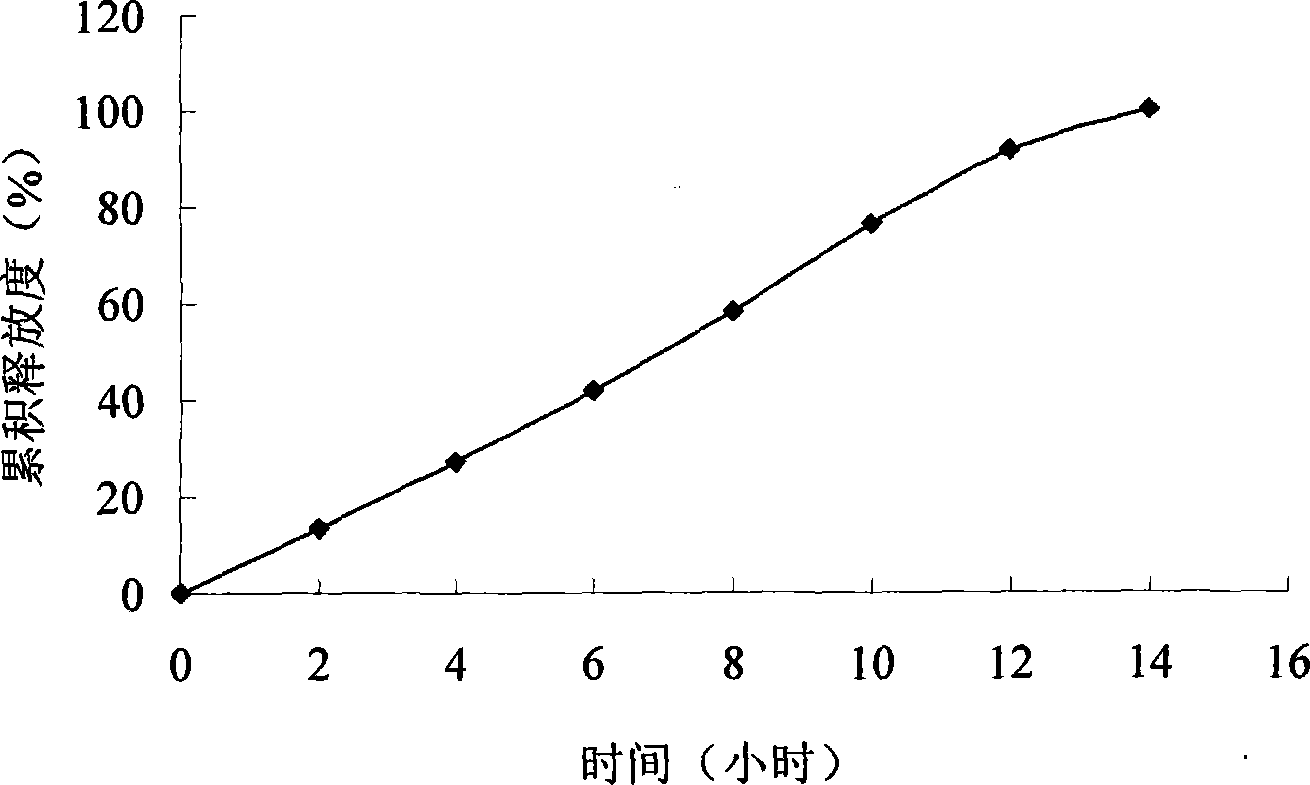
Embodiment 1
[0047] prescription:
[0048] (1) Drug-containing layer
[0049] Pitavastatin Calcium 0.5%
[0050] Dicalcium Phosphate 40%
[0051] Sodium bicarbonate 2%
[0053] Polyoxyethylene 19%
[0054] Magnesium Stearate 0.5%
[0055] Appropriate amount of water
[0056] (2) Push layer
[0057] Polyoxyethylene 25%
[0059] Magnesium Stearate 0.5%
[0060] Proper amount of ethanol
[0061] (3) Coating layer
[0062] Cellulose Acetate 95%
[0063] Macrogol 4000 5%
[0064] Proper amount of ethanol
[0065] Preparation:
[0066] (1) According to the above prescription, pitavastatin calcium is mixed with basic auxiliary materials, osmotic pressure active substances, penetration enhancers and other auxiliary materials and then granulated to obtain drug-containing layer granules; the penetration enhancer, osmotic pressure active substances and other auxiliary materials are mixed After granulation, the push layer granu...
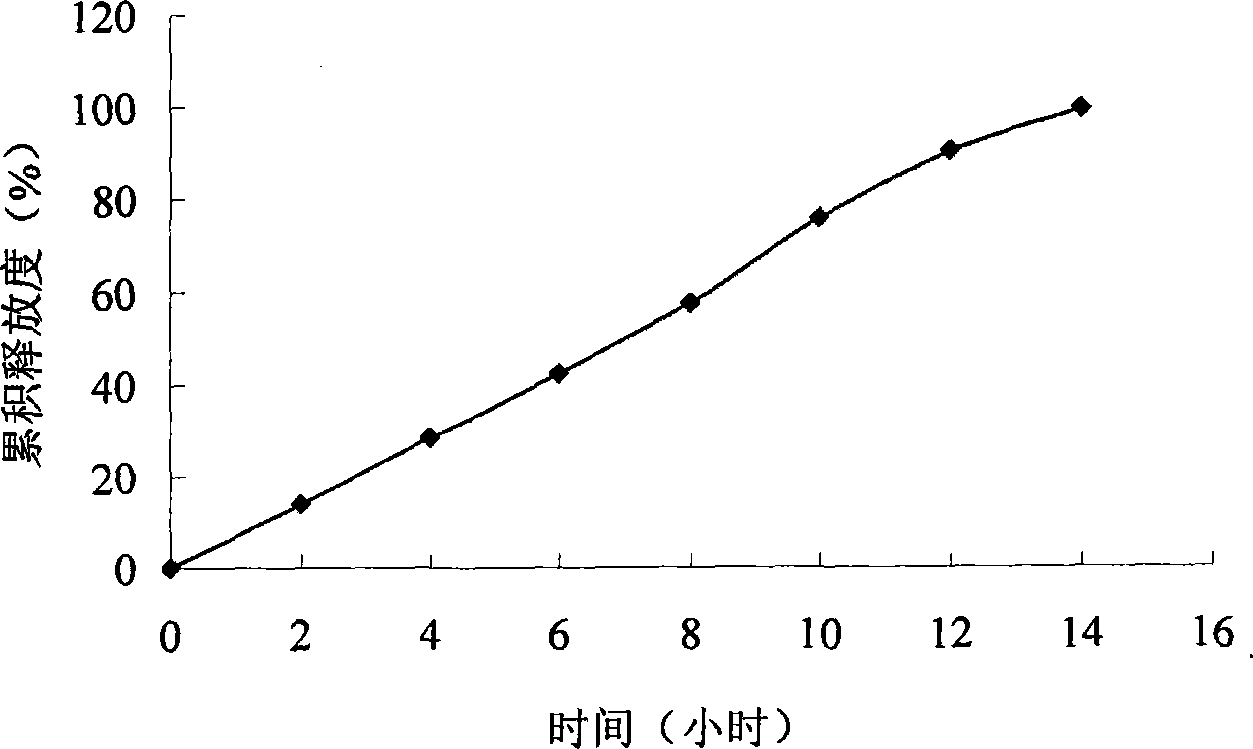
Embodiment 2
[0071] prescription:
[0072] (1) Drug-containing layer
[0073] Pitavastatin Calcium 1%
[0074] Calcium Carbonate 15%
[0076] Carbopol 35%
[0077] Lactose 6.5%
[0078] Magnesium Stearate 0.5%
[0079] Povidone K30 3%
[0080] Proper amount of ethanol
[0081] (2) Push layer
[0082] Polyoxyethylene 19%
[0083] Sodium Chloride 12%
[0084] Hypromellose 2.5%
[0085] Magnesium Stearate 0.5%
[0086] Proper amount of ethanol
[0087] (3) Coating layer
[0088] Cellulose acetate 91.3%
[0089] Triethyl Citrate 6.3%
[0090] Macrogol 400 2.4%
[0091] Proper amount of ethanol
[0092] Preparation:
[0093] (1) According to the above prescription, pitavastatin calcium is mixed with basic auxiliary materials, osmotic pressure active substances, penetration enhancers and other auxiliary materials and then granulated to obtain drug-containing layer granules; the penetration enhancer, osmotic pressure active substances and other auxili...
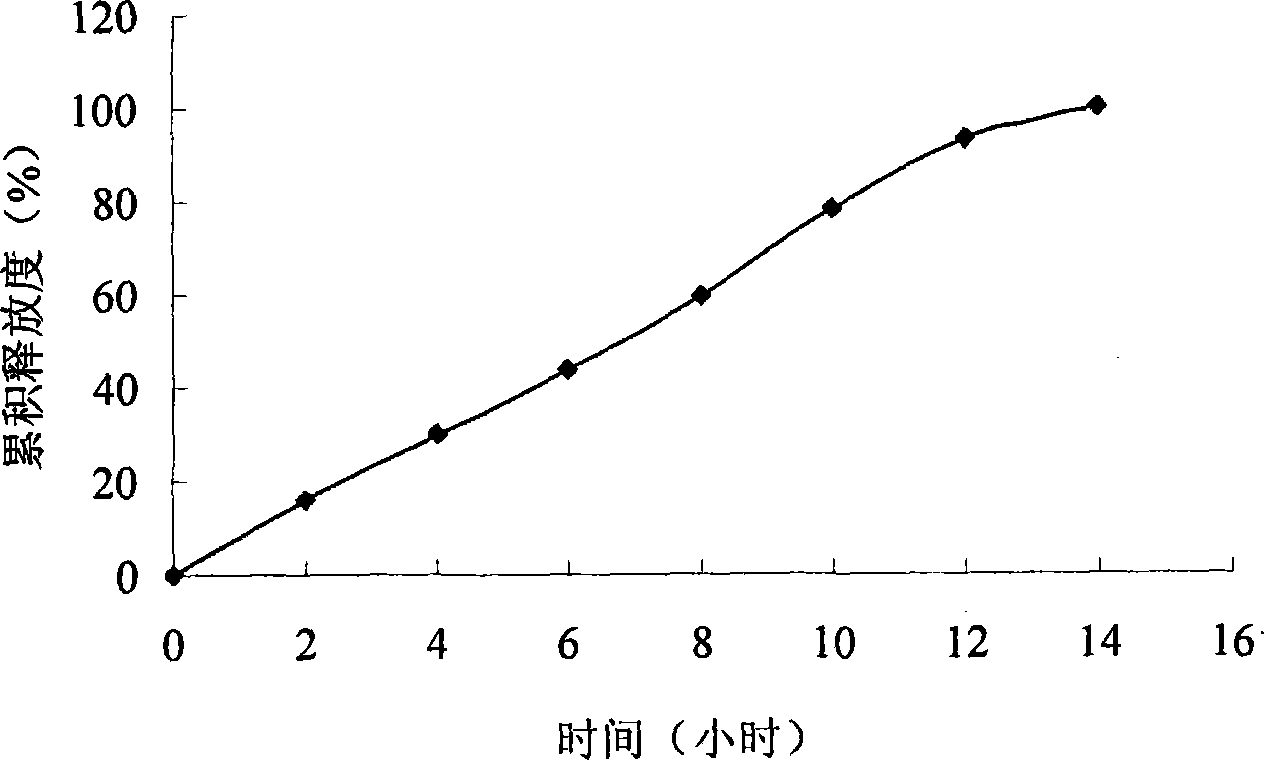
Embodiment 3
[0098] prescription:
[0099] (1) Drug-containing layer
[0100] Pitavastatin Calcium 0.8%
[0101] Sodium bicarbonate 10%
[0102] Sodium Chloride 12%
[0103] Polyoxyethylene 36%
[0104] Magnesium Stearate 0.6%
[0105] Proper amount of ethanol
[0106] (2) Push layer
[0107] Sodium Starch Carboxymethyl 30.5%
[0108] Sodium Chloride 9.5%
[0109] Magnesium Stearate 0.6%
[0110] Proper amount of ethanol
[0111] (3) Coating layer
[0112] Cellulose Acetate 95%
[0113] Macrogol 1500 5%
[0114] Proper amount of acetone
[0115] Preparation:
[0116] (1) According to the above prescription, pitavastatin calcium is mixed with basic auxiliary materials, osmotic pressure active substances, penetration enhancers and other auxiliary materials and then granulated to obtain drug-containing layer granules; the penetration enhancer, osmotic pressure active substances and other auxiliary materials are mixed After granulation, the push layer granules are obtained; the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com