Azithromycin sustained-release pellet capsule and preparation method thereof
A technology for azithromycin and sustained-release pellets, which is applied in the field of azithromycin sustained-release pellets and its preparation, can solve problems such as increased incidence, and achieve the effects of low material loss, reduced side effects, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
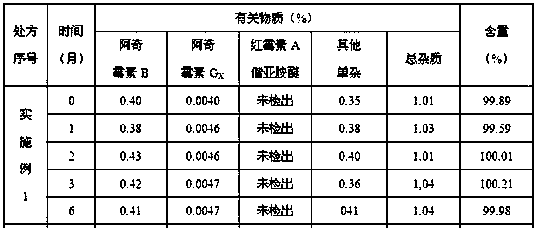
Embodiment 1
[0033] Ball core:
[0034] Prescription Composition Prescription Amount
[0035] Azithromycin 500g
[0036] Croscarmellose Sodium 5g
[0037] Lactose 62.5g
[0038] Macrogol 6000 5g
[0039] Appropriate amount of purified water
[0040] Slow-release coating:
[0041] Prescription Composition Prescription Amount
[0042] Eurdragit RL 30D 35g
[0043] Triethyl citrate 4g
[0045] Appropriate amount of purified water
[0046] Coating weight gain 20%~25%
[0047] Make 1000 capsules.
[0048] Preparation of pellets: Pass the azithromycin raw material through a 100-mesh sieve, weigh the prescribed amount of azithromycin, croscarmellose sodium, and lactose, mix them evenly in an extruder, and use polyethylene glycol 6000 aqueous solution for bonding To make wet and soft materials, put the soft materials in an extruder to extrude pellets. The sieve aperture is preferably 1mm, and the extrusion speed is 20~30rpm. 1000rpm. The spheronized pell...
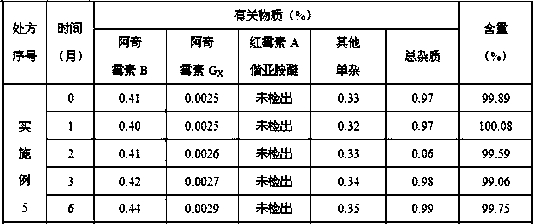
Embodiment 2
[0052] Ball core:
[0053] Prescription Composition Prescription Amount
[0054] Azithromycin 500g
[0055] Croscarmellose Sodium 31.25g
[0056] Lactose 5g
[0057] Macrogol 6000 5g
[0058] Appropriate amount of purified water
[0059] Slow-release coating:
[0060] Prescription Composition Prescription Amount
[0061] Eurdragit RL 30D 35g
[0062] Triethyl citrate 4g
[0064] Appropriate amount of purified water
[0065] Coating weight gain 20%~25%
[0066] Make 1000 capsules.
[0067] Preparation of pellets: Pass the azithromycin raw material through a 100-mesh sieve, weigh the prescribed amount of azithromycin, croscarmellose sodium, and lactose, mix them evenly in an extruder, and use polyethylene glycol 6000 aqueous solution for bonding To make wet and soft materials, put the soft materials in an extruder to extrude pellets. The sieve aperture is preferably 1mm, and the extrusion speed is 20~30rpm. 1000rpm. The spheronized pel...
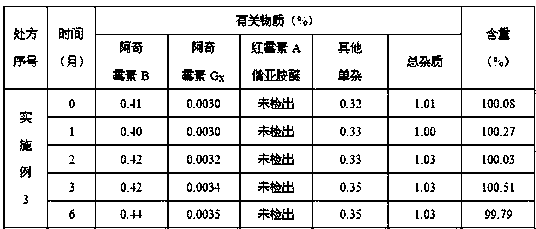
Embodiment 3
[0071] Ball core:
[0072] Prescription Composition Prescription Amount
[0073] Azithromycin 500g
[0074] Croscarmellose Sodium 5g
[0075] Lactose 33.75g
[0076] Macrogol 6000 18.125g
[0077] Appropriate amount of purified water
[0078] Slow-release coating:
[0079] Prescription Composition Prescription Amount
[0080] Eurdragit RL 30D 35g
[0081] Triethyl citrate 4g
[0083] Appropriate amount of purified water
[0084] Coating weight gain 20%~25%
[0085] Make 1000 capsules.
[0086] Preparation of pellets: Pass the azithromycin raw material through a 100-mesh sieve, weigh the prescribed amount of azithromycin, croscarmellose sodium, and lactose, mix them evenly in an extruder, and use polyethylene glycol 6000 aqueous solution for bonding To make wet and soft materials, put the soft materials in an extruder to extrude pellets. The sieve aperture is preferably 1mm, and the extrusion speed is 20~30rpm. 1000rpm. The spheronize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com