Simvastatin osmotic pump preparation and preparation method thereof
A technology of simvastatin and osmotic pump tablets, which is applied in the field of simvastatin sustained and controlled release preparations, and can solve the problems that the sustained release effect needs to be further improved, the preparation cost is high, and the drug release is not ideal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
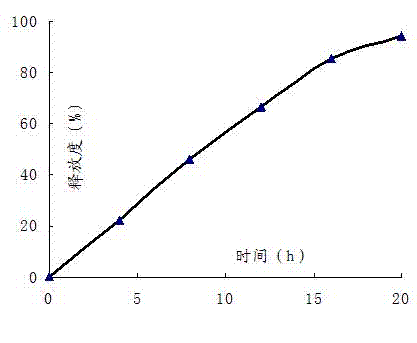
Embodiment 1
[0051]
[0052] Controlled Release Film Coat Composition Dosage per tablet (mg) Proportion(%) Cellulose acetate 7.2 90 Polyethylene glycol-1500 0.4 5 triethyl citrate 0.4 5
[0053] Preparation Process:
[0054] Pass the simvastatin, polyoxyethylene (2 million), sucrose, lactose, dextrin, microcrystalline cellulose, povidone, sodium lauryl sulfate, and vitamin C to be prepared through an 80-mesh sieve and mix thoroughly. Add ethanol to make the soft material; the soft material is granulated with a 30-mesh sieve; after drying, the granules are sieved with a 20-mesh sieve, and then magnesium stearate is added and mixed well; the prepared granules are compressed with a tablet machine; the controlled-release film coating is formed The raw material is dissolved in acetone, and the tablet core is coated with a controlled-release film, and the weight of the coating is increased by 6% to 8%. A small hole of 0.4-0.8mm is punched on both side...
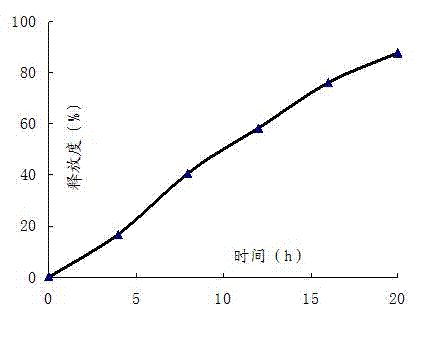
Embodiment 2
[0056]
[0057] Controlled Release Film Coat Composition Dosage per tablet (mg) Proportion(%) Cellulose acetate 6.4 80 Polyethylene glycol-1500 1.6 20
[0058] Preparation Process:
[0059]Pass the simvastatin, polyoxyethylene (2 million), sucrose, lactose, dextrin, microcrystalline cellulose, povidone, and vitamin C to be prepared through an 80-mesh sieve, mix them well, and set aside; polysorbate- 80 plus ethanol to dissolve and add the above dry powder to make soft material; the soft material is granulated with a 30-mesh sieve; after drying, 20-mesh sieve is granulated, and then magnesium stearate is added and mixed; the prepared granules are compressed with a tablet machine; Dissolve the raw materials of the controlled-release film coating in acetone, and coat the tablet core with a controlled-release film. The weight of the coating increases by 6% to 8%. A 0.4 A small hole of ~0.8mm.
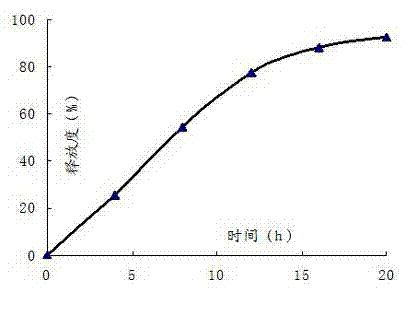
Embodiment 3
[0061]
[0062] Controlled Release Film Coat Composition Dosage per tablet (mg) Proportion(%) Cellulose acetate 6.4 80 Polyethylene glycol-1500 1.6 20
[0063] Preparation Process:
[0064] Pass the simvastatin, polyoxyethylene (2 million), sucrose, lactose, dextrin, microcrystalline cellulose, povidone, sodium lauryl sulfate, and vitamin C to be prepared through an 80-mesh sieve and mix thoroughly. Add ethanol to make the soft material; the soft material is granulated with a 30-mesh sieve; after drying, the granules are sieved with a 20-mesh sieve, and then magnesium stearate is added and mixed well; the prepared granules are compressed with a tablet machine; the controlled-release film coating is formed The raw material is dissolved in acetone, and the tablet core is coated with a controlled-release film, and the weight of the coating is increased by 6% to 8%. A small hole of 0.4-0.8mm is punched on both sides of the controlled-release film...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ion source temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com