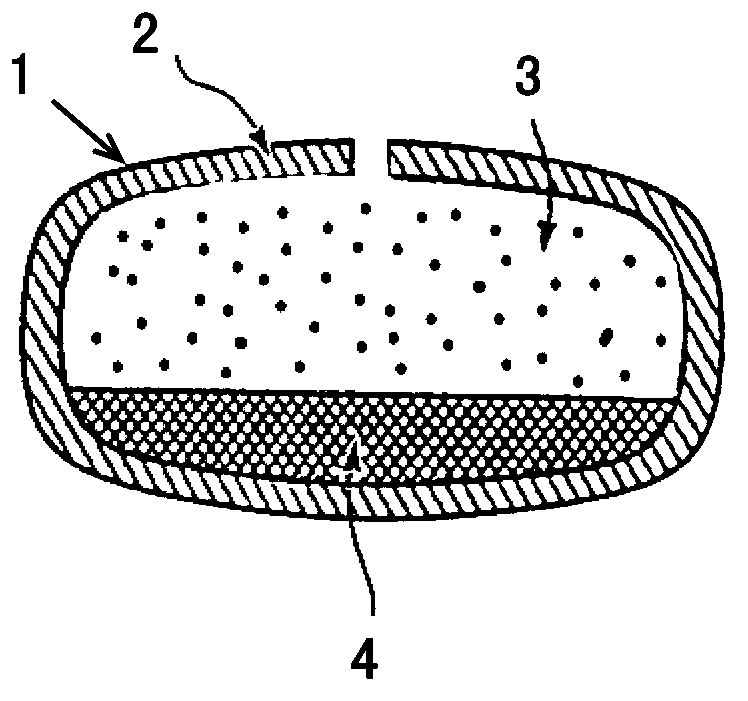
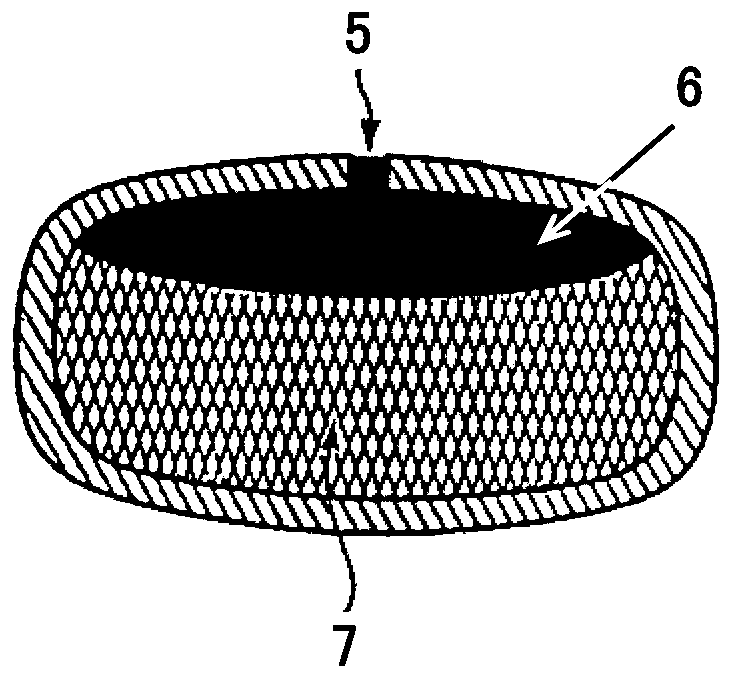
Phencynonate hydrochloride double-layer osmotic pump controlled release tablet and preparation method thereof
A technology of phencyclonyl hydrochloride, osmotic pump controlled release, applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc. Poor etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Prescription composition: see Table 1.
[0080] Preparation process: see Figure 12 .
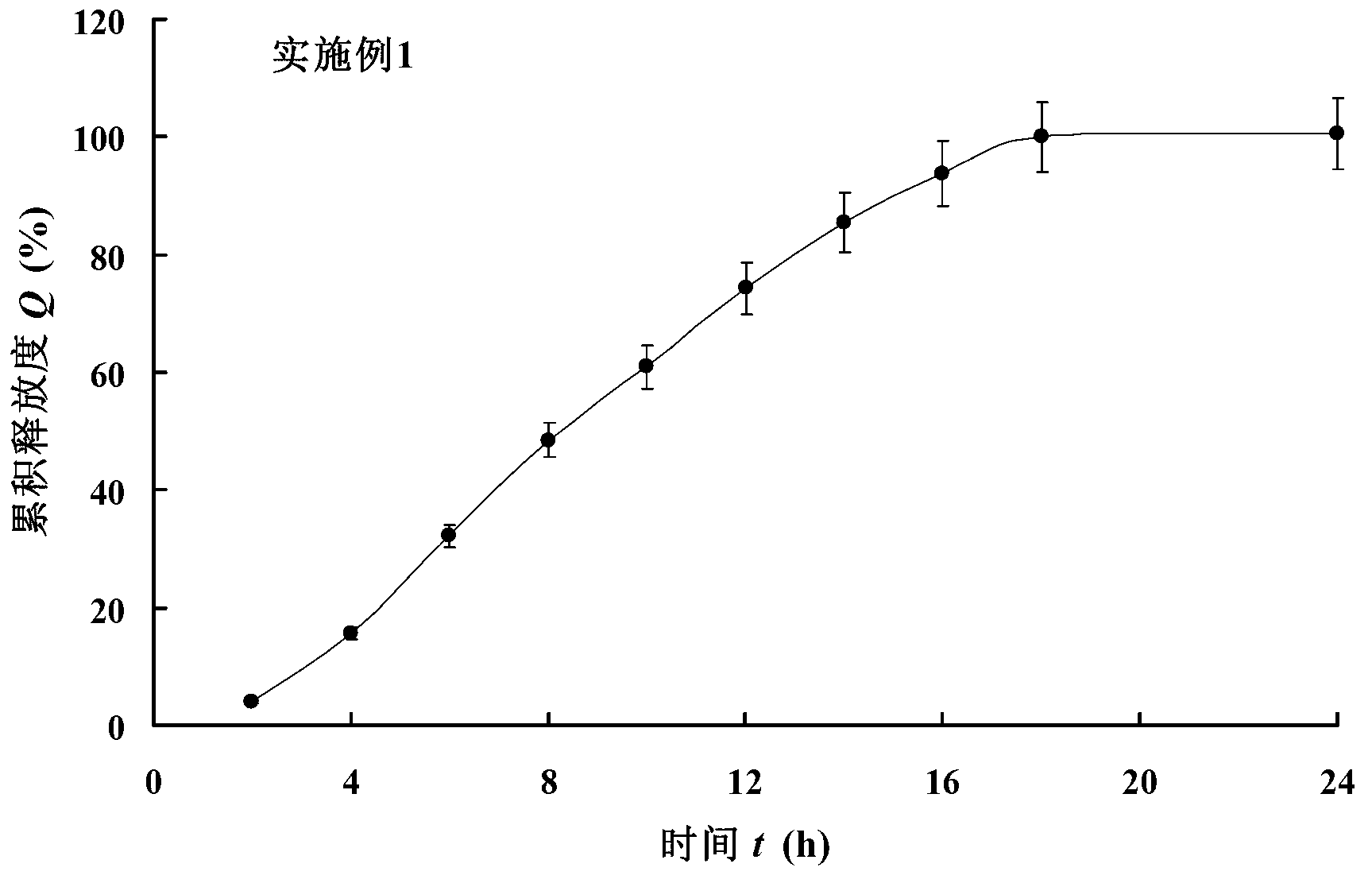
[0081] Dissolution determination method: get the phencyclonyl hydrochloride double-layer osmotic pump controlled-release tablet sample prepared in this embodiment, according to the release determination method (Chinese Pharmacopoeia 2010 edition two appendix X D first method), adopt the dissolution determination method ( Chinese Pharmacopoeia 2010 Edition, Part II Appendix X C Method 2), using 900mL of water as the release medium, the rotation speed is 50 revolutions per minute, the medium temperature is 37°C±0.5°C, operated according to the law, in 2h, 4h, 6h, 8h, 10h , 12h, 14h, 16h, 18h, and 24h, respectively take 4mL of the solution, filter it with a 0.45μm filter membrane, and take the subsequent filtrate as the test solution. Immediately after each sampling, add water of the same temperature and volume to each dissolution vessel. Another appropriate amount of phencyclonyl hy...
Embodiment 2
[0083] Prescription composition: see Table 1.
[0084] Preparation process: see Figure 13 .
[0085] Release measurement method: same as in Example 1, in vitro cumulative 24h release percentage-time curve see Figure 4 .
Embodiment 3
[0087] Prescription composition: see Table 1.
[0088] Preparation process: see Figure 14 .
[0089] Release measurement method: same as in Example 1, in vitro cumulative 24h release percentage-time curve see Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com