Self-assembled composite membrane controlled sustained-release preparation and preparation method thereof
A technology for sustained-release preparations and complexes, applied in the field of medicine, can solve problems such as high cost and complex process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Theophylline Sustained Release Tablets
[0038] Theophylline 15.0g
[0039] Chitosan 7.1g
[0040] Sodium alginate 7.1g
[0041] Microcrystalline Cellulose 0.9g
[0042] Magnesium stearate 0.03g
[0043]
[0044] Makes 100 pieces
[0045] Preparation Process:
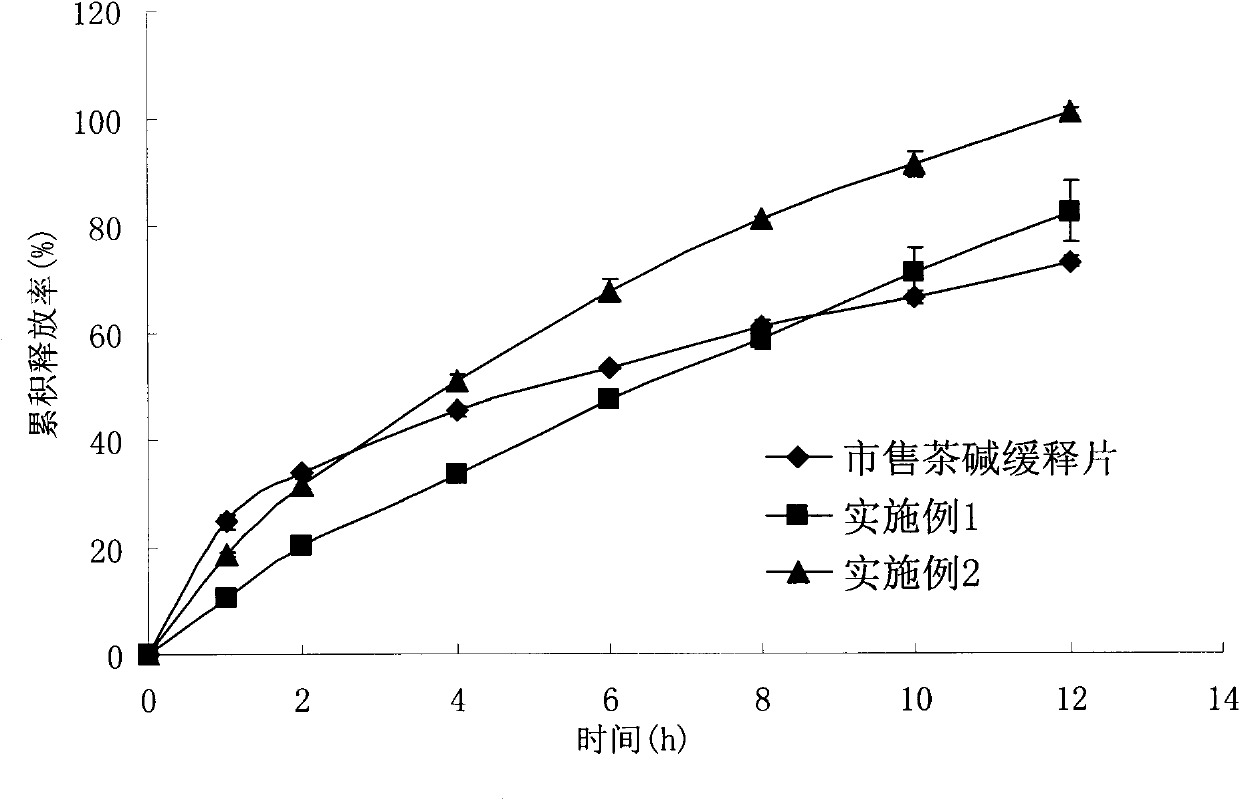
[0046] The drug and auxiliary materials are sieved through 100 sieves, mixed uniformly in equal increments, and compressed into tablets with an average tablet weight of 300 mg. The chitosan is chitosan with a molecular weight of 400kDa and a deacetylation degree of 86.5%. The sodium alginate is sodium alginate with a molecular weight of 400kDa and a G / M ratio of 40:60. The dissolution results of the preparation in the simulated gastrointestinal fluid are shown in the appendix figure 2 (dissolution conditions are attached figure 1 ).
[0047]Theophylline sustained-release tablets made by the above-mentioned method meet the requirements of the relevant testing items st...
Embodiment 2
[0049] Theophylline Sustained Release Tablets
[0050] Theophylline 10.0g
[0051] Chitosan 6.6g
[0052] Sodium alginate 6.6g
[0053] Lactose 6.0g
[0054] Microcrystalline Cellulose 0.9g
[0055] Magnesium stearate 0.03g
[0056]
[0057] Makes 100 pieces
[0058] Preparation Process:
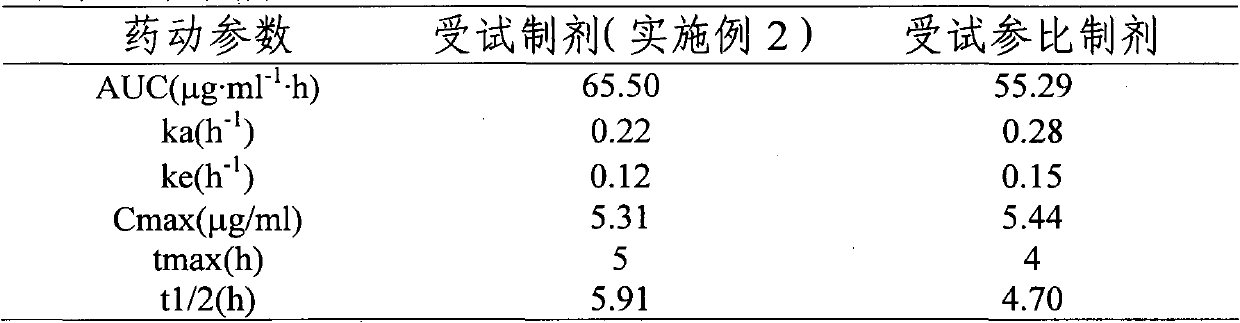
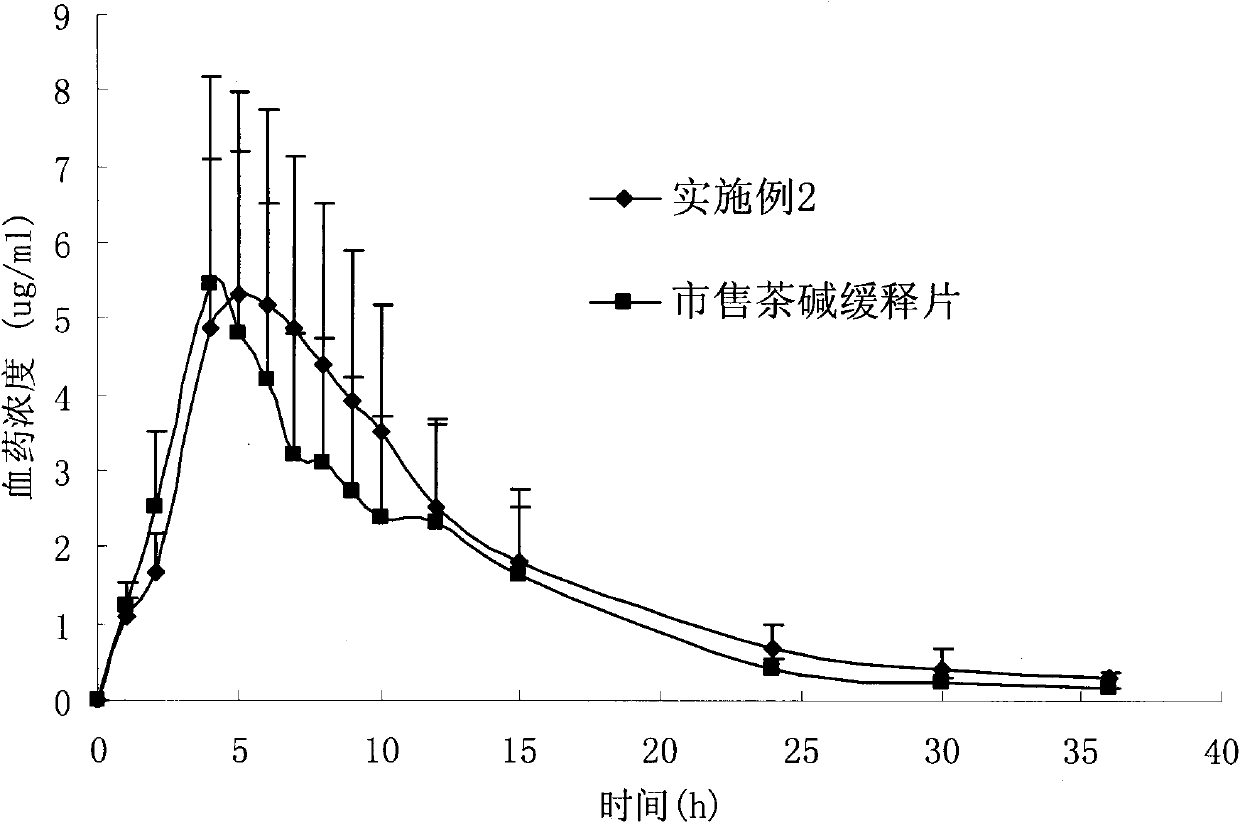
[0059] Pass the drug and auxiliary materials through 100 sieves, mix them uniformly in equal increments, and compress them into tablets with an average tablet weight of 300 mg. The chitosan is chitosan with a molecular weight of 400kDa and a deacetylation degree of 86.5%. The sodium alginate is sodium alginate with a molecular weight of 350kDa and a G / M ratio of 40:60. The lactose in the prescription can be replaced by any one of starch, polyethylene glycol 4000, polyethylene glycol 6000, sucrose, mannitol, and polyvinylpyrrolidone. The dissolution results of the preparation in the simulated gastrointestinal fluid and the comparison with the ...
Embodiment 3
[0062] Theophylline Sustained Release Tablets / Granules
[0063] Theophylline 15.0g
[0064] Chitosan 3.6g
[0065] Sodium Carboxymethyl Cellulose 10.5g
[0066] Microcrystalline Cellulose 0.9g
[0067] Magnesium stearate 0.03g
[0068]
[0069] Makes 100 pieces
[0070] Preparation Process:
[0071] Pass the drug and auxiliary materials through a 100-mesh sieve, mix theophylline, chitosan, sodium carboxymethylcellulose and microcrystalline cellulose in equal amounts, and use 70% ethanol as a soft material to pass through a 14-mesh sieve Granules, dried in an oven at 30°C, granulated through a 12-mesh sieve to obtain sustained-release granules, or granulated by extrusion, spheronization, etc., mixed with magnesium stearate, and compressed into average tablets Tablets weighing 300 mg were obtained as sustained-release tablets. The chitosan is chitosan with a molecular weight of 200kDa and a deacetylation degree of 86.5%. The molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com