Posaconazole double-layer osmotic pump controlled release tablet and preparation method thereof
A posaconazole double-layer, osmotic pump controlled release technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can avoid the fluctuation of blood drug concentration, good correlation between in vitro and in vivo, and stable blood drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
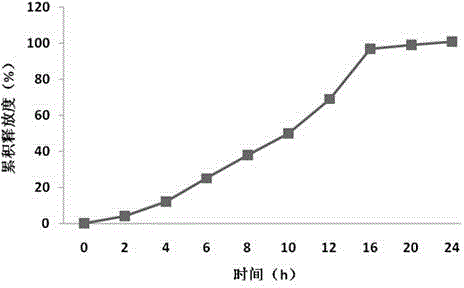
Embodiment 1
[0053] Preparation Process:
[0054] 1) Posaconazole pretreatment
[0055] The posaconazole raw material is ultrafinely pulverized, with an average particle size of 0.5-10 μm;
[0056] 2) Preparation of drug-containing layer particles
[0057] Mix posaconazole superfine powder, polyoxyethylene with a molecular weight of 100,000-2 million, an osmotic pressure active substance, and an adhesive in a mixer to obtain drug-containing layer particles, add a lubricant, and set aside;
[0058] 3) Preparation of booster layer particles
[0059] Polyoxyethylene with a molecular weight of 4-8 million, osmotic pressure active substances, and colorants are wet-granulated with 70% ethanol solution, and dried in a fluidized bed, but not limited to a fluidized bed, to obtain booster layer granules, and lubricants are added. spare;
[0060] 4) Preparation of double-layer tablet core
[0061] A double-layer tablet press is used to compress the posaconazole double-layer tablet. First, the dr...
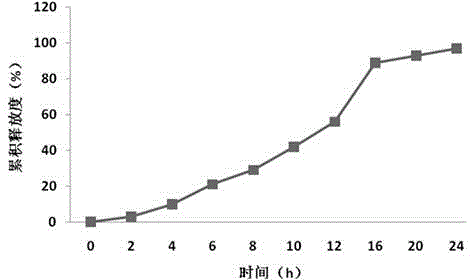
Embodiment 2
[0067] Preparation process: (same as Example 1)
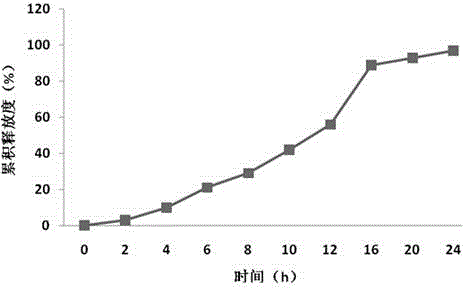
Embodiment 3
[0069] Preparation process: (same as Example 1)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com