PEG site-modified asparaginase injection
A technology of asparaginase injection and asparaginase, which is applied in the field of injection of polyethylene glycol N-terminal fixed-point modification of asparaginase, to achieve the effects of stable blood drug concentration, uniform modified product and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation and analysis of pegylated asparaginase with N-terminal site-directed modification
preparation example 1
[0056] Preparation Example 1, the pegylated asparaginase with N-terminal site-specific modification of the present invention is prepared, purified and identified by the following methods:
[0057] 1. Preparation of pegylated asparaginase with N-terminal site-directed modification
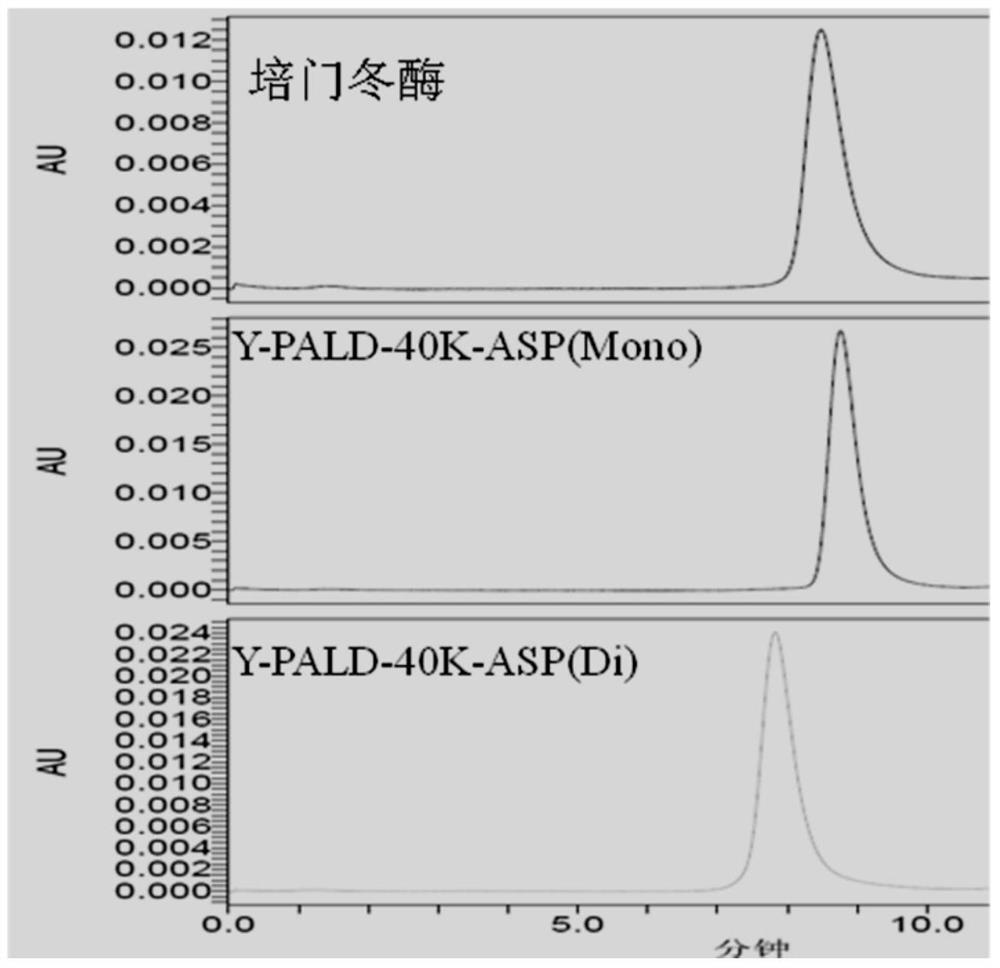
[0058] Dissolve L-asparaginase (from Changzhou Qianhong Biochemical Pharmaceutical Co., Ltd.) with 40mM acetic acid-sodium acetate buffer solution of pH5.0 to prepare a 20mg / mL solution, and PEG uses Y-PALD-40K (purchased from Beijing Jiankai Technology Co., Ltd.), according to the molar ratio of asparaginase:PEG:sodium cyanoborohydride of 1:4:200, reacted at 4°C for 12h, and then added 1M glycine to terminate the reaction. The monomodified product Y-PALD-40K-ASP (Mono) and the dimodified product Y-PALD-40K-ASP (Di) were prepared.
[0059] 2. Purification of pegylated asparaginase with N-terminal site-directed modification
[0060] Chromatography conditions: Q ion exchange column (purchased from G...
preparation example 2
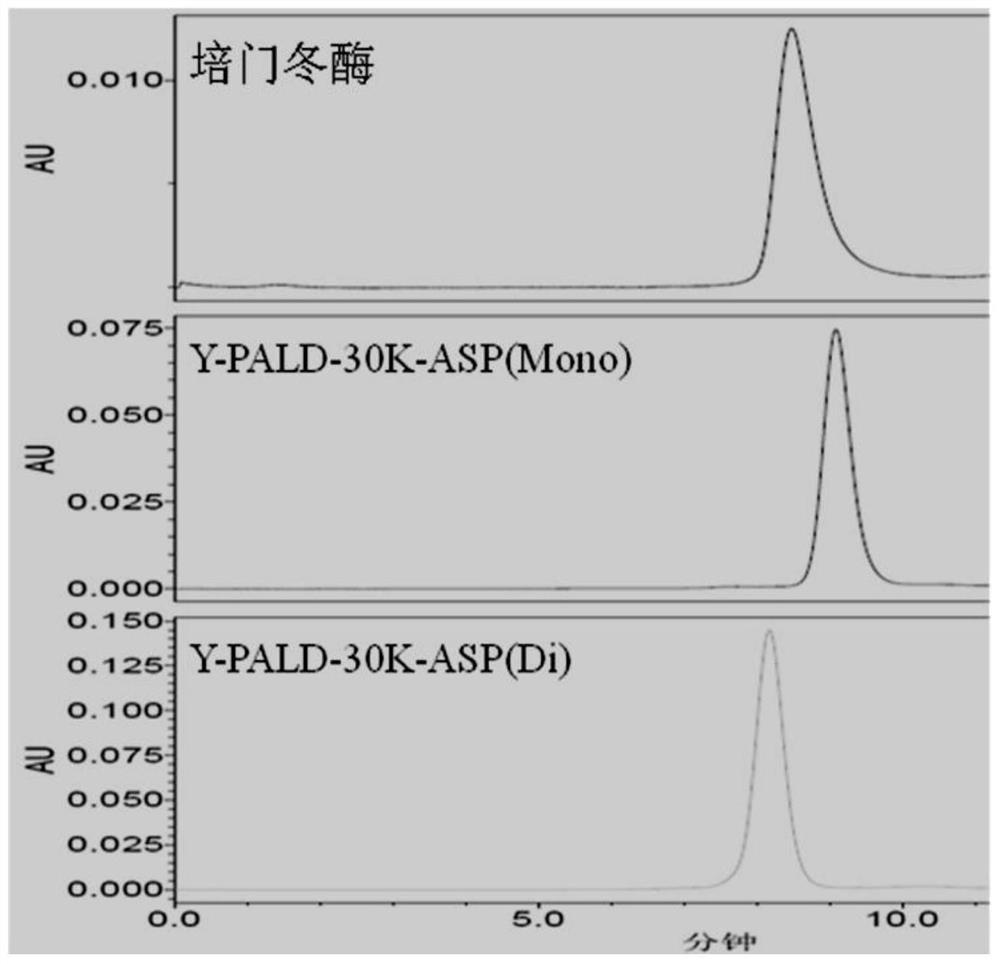
[0074] The activity and purity of the monomodified and dimodified products prepared in Preparation Example 2 were not significantly different from the monomodified and dimodified products obtained in Preparation Example 1. Y-PALD-30K-ASP (Mono) and Y-PALD- The purity of 30K-ASP(Di) was higher than 98%.
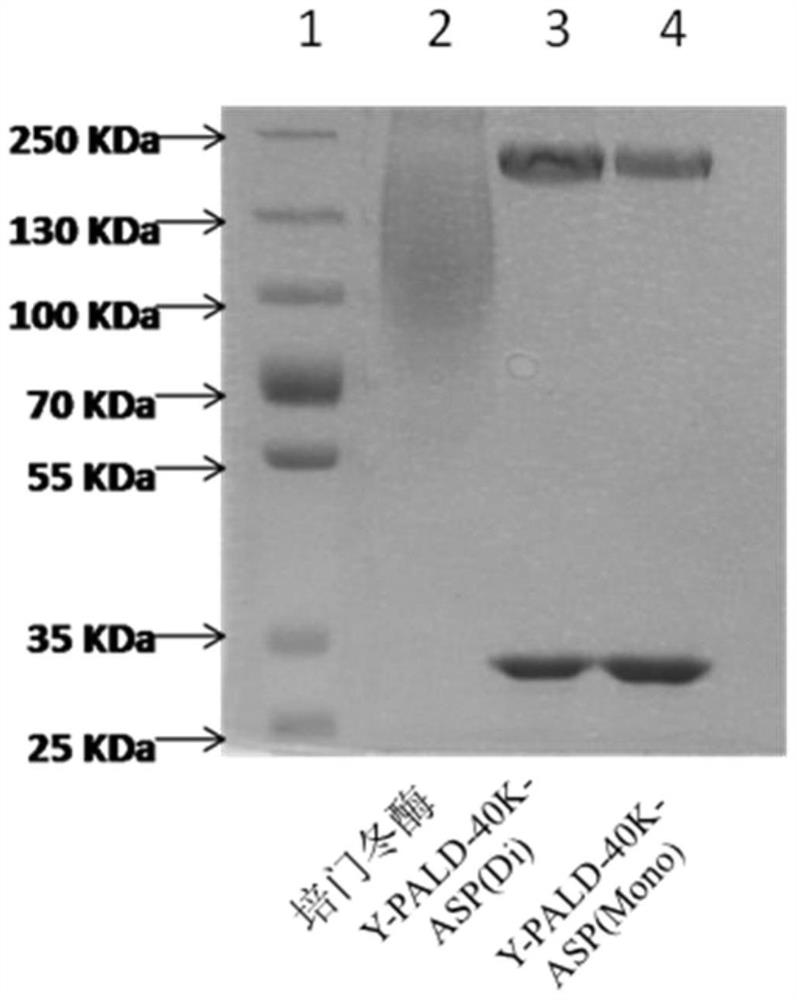
[0075] Depend on Figure 2a , 2b It can be seen that the modified product Y-PALD-40K-ASP (Mono) and Y-PALD-40K-ASP (Di), Y-PALD-30K-ASP (Mono) and Y-PALD-30K-ASP (Di) Electrophoretic bands are very uniform. Compared with the similar product on the domestic market—pegaspargase (purchased from Jiangsu Hengrui Medicine Co., Ltd.), the uniformity has been greatly improved.
[0076] Figure 2aThe 3rd and 4th lanes show two uniform bands, and the molecular weight corresponding to the first band in the 3rd and 4th lanes is about 200KDa, because PEG molecules will bind a large amount of water molecules in the solution, making Its apparent molecular weight is about 4 times its theor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com